CyclomulberrinCAS# 19275-51-5 |
Quality Control & MSDS
Number of papers citing our products

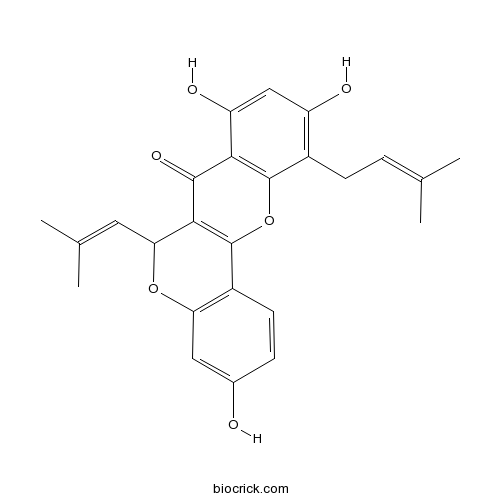
Chemical structure

3D structure
| Cas No. | 19275-51-5 | SDF | Download SDF |
| PubChem ID | 11742872 | Appearance | Yellow powder |
| Formula | C25H24O6 | M.Wt | 420.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,8,10-trihydroxy-11-(3-methylbut-2-enyl)-6-(2-methylprop-1-enyl)-6H-chromeno[4,3-b]chromen-7-one | ||
| SMILES | CC(=CCC1=C(C=C(C2=C1OC3=C(C2=O)C(OC4=C3C=CC(=C4)O)C=C(C)C)O)O)C | ||
| Standard InChIKey | SYFDWXWLRGHYAJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H24O6/c1-12(2)5-7-15-17(27)11-18(28)21-23(29)22-20(9-13(3)4)30-19-10-14(26)6-8-16(19)25(22)31-24(15)21/h5-6,8-11,20,26-28H,7H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Cyclomulberrin exhibits potent inhibition of human PLC/PRF/5 and KB cells in-vitro. 2. Cyclomulberrin shows strong inhibition of arachidonic acid (AA)- and collagen-induced platelet aggregation. 3. Cyclomulberrin also shows slight but significant antiplatelet effects on the aggregation induced by PAF. 4. Cyclomulberrin enhances cell viability in a dose-dependent manner against sodium nitroprusside-induced cell death in neuroblastoma SH-SY5Y cells. |

Cyclomulberrin Dilution Calculator

Cyclomulberrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3781 mL | 11.8906 mL | 23.7812 mL | 47.5624 mL | 59.453 mL |
| 5 mM | 0.4756 mL | 2.3781 mL | 4.7562 mL | 9.5125 mL | 11.8906 mL |
| 10 mM | 0.2378 mL | 1.1891 mL | 2.3781 mL | 4.7562 mL | 5.9453 mL |
| 50 mM | 0.0476 mL | 0.2378 mL | 0.4756 mL | 0.9512 mL | 1.1891 mL |
| 100 mM | 0.0238 mL | 0.1189 mL | 0.2378 mL | 0.4756 mL | 0.5945 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cudraflavone B
Catalog No.:BCN8067
CAS No.:19275-49-1
- 3-Isomangostin
Catalog No.:BCN1214
CAS No.:19275-46-8
- Lopinavir
Catalog No.:BCC3621
CAS No.:192725-17-0
- C527
Catalog No.:BCC3972
CAS No.:192718-06-2
- PD 161570
Catalog No.:BCC7765
CAS No.:192705-80-9
- H-DL-HoSer-OH
Catalog No.:BCC3242
CAS No.:1927-25-9
- 9-Benzoylcarbazole
Catalog No.:BCC8799
CAS No.:19264-68-7
- PPADS tetrasodium salt
Catalog No.:BCC6725
CAS No.:192575-19-2
- 11α-Hydroxycanrenone
Catalog No.:BCC8433
CAS No.:192569-17-8
- 9alpha,11,12-Trihydroxydrim-7-en-6-one
Catalog No.:BCN7388
CAS No.:192566-65-7
- Ergosta-4,6,8(14),22-tetraen-3-one
Catalog No.:BCN1183
CAS No.:19254-69-4
- Lomeguatrib
Catalog No.:BCC1133
CAS No.:192441-08-0
- ES 936
Catalog No.:BCC6102
CAS No.:192820-78-3
- 2-Amino-2'-nitrodiphenyl sulfide
Catalog No.:BCC8522
CAS No.:19284-81-2
- LY310762
Catalog No.:BCC5052
CAS No.:192927-92-7
- Ximelagatran
Catalog No.:BCC6382
CAS No.:192939-46-1
- ZM 323881 HCl
Catalog No.:BCC5098
CAS No.:193000-39-4
- ZM323881
Catalog No.:BCC2073
CAS No.:193001-14-8
- CTS-1027
Catalog No.:BCC1502
CAS No.:193022-04-7
- Cardamonin
Catalog No.:BCN1184
CAS No.:19309-14-9
- Otamixaban
Catalog No.:BCC1827
CAS No.:193153-04-7
- cis-Moschamine
Catalog No.:BCN3901
CAS No.:193224-24-7
- 1-O-Acetyl-6alpha-O-(2-methylbutyryl)britannilactone
Catalog No.:BCN7747
CAS No.:1932687-71-2
- Lonafarnib
Catalog No.:BCC2331
CAS No.:193275-84-2
Protection of prenylated flavonoids from Mori Cortex Radicis (Moraceae) against nitric oxide-induced cell death in neuroblastoma SH-SY5Y cells.[Pubmed:22297755]
Arch Pharm Res. 2012 Jan;35(1):163-70.
Seven prenylated flavanoids, licoflavone C (1), Cyclomulberrin (2), neocyclomorusin (3), sanggenon I (4), morusin (5), kuwanon U (6) and kuwanon E (7), and three 2-arylbenzofurans, moracin P (8), moracin O (9), and mulberrofuran Q (10) were isolated from the MeOH extract of Mori Cortex Radicis. Among these, compounds 2-7 enhanced cell viability in a dose-dependent manner against sodium nitroprusside-induced cell death in neuroblastoma SH-SY5Y cells, which was measured by MTT reduction assay (EC(50) values of 4.4, 5.6, 8.0, 6.4, 8.7, and 11.9 mug/mL, respectively). Among 10 compounds, C-3 prenylated flavones (2, 3, and 5) and prenylated flavanones (4, 6, and 7) showed cell protection. However, compound 1 which lacks the prenyl group at C-3 and three 2-arylbenzofurans (8-10) did not show protective effect. The order of cell protection was as follow: C-3 prenylated flavones (2, 3, and 5) > prenylated flavanones (4, 6, and 7) > 2-arylbenzofurans (8-10) and flavone (1). From this result, we show that some prenylated flavones and flavanones might protect neuronal cells against nitrosative stress-mediated cell death. Even though further evaluations are necessary in vitro and in vivo study, we carefully suggest that some prenylated flavonoids from Mori Cortex Radicis might protect neuronal cells from neurodegenerative diseases.
Antiplatelet activity of some prenylflavonoids.[Pubmed:8435100]
Biochem Pharmacol. 1993 Jan 26;45(2):509-12.
Eight naturally occurring prenylflavonoids were tested for their antiplatelet activities in rabbit platelet suspension. Cyclomorusin and artomunoxanthone showed strong inhibition of platelet-activating factor (PAF; 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) induced platelet aggregation. Cyclomulberrin, dihydroisocycloartomunin, cyclocommunol and cyclocommunin showed strong inhibition of arachidonic acid (AA)- and collagen-induced platelet aggregation. Cyclomorusin also inhibited markedly collagen-induced platelet aggregation. Cyclocommunin, dihydroisocycloartomunin and Cyclomulberrin also showed slight but significant antiplatelet effects on the aggregation induced by PAF. Of the compounds tested, cyclocommunin exhibited the most potent inhibition of platelet aggregation induced by collagen (IC50 = 14.4 microM) and AA (IC50 = 12.5 microM). Thromboxane B2 formation caused by AA was suppressed by cyclocommunin and artomunoxanthone.
Gamma-pyrone compounds as potential anti-cancer drugs.[Pubmed:7903365]
J Pharm Pharmacol. 1993 Sep;45(9):791-4.
The gamma-pyrones, artomunoxanthotrione epoxide, cyclocommunol, Cyclomulberrin, and cyclocommunin exhibited potent inhibition of human PLC/PRF/5 and KB cells in-vitro. Dihydroisocycloartomunin showed significant and potent inhibition of human PLC/PRF/5 and KB cells in-vitro, respectively. Cyclomorusin, dihydrocycloartomunin and artomunoxanthone showed significant inhibition of KB cells in-vitro. Based on the above finding and the reported antileukaemic activity of xanthone psorospermin, a series of natural gamma-pyrones was prepared and the inhibition of human PLC/PRF/5 and KB cells in-vitro was measured. Structure-activity analysis indicated the epoxide group substituted at 3-hydroxyl and 2,6-; 3,6-; and 3,5-dihydroxyl xanthone enhanced the anti-tumour activity. The epoxide group substituted at the 6-hydroxyl group of 1,6-dihydroxyxanthone did not show anti-tumour activity.


