LomeguatribMGMT inhibitor CAS# 192441-08-0 |
- 5-Azacytidine
Catalog No.:BCC1130
CAS No.:320-67-2
- Zebularine
Catalog No.:BCC1136
CAS No.:3690-10-6
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
- Nanaomycin A
Catalog No.:BCC3611
CAS No.:52934-83-5
- SGI-110
Catalog No.:BCC2221
CAS No.:929901-49-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 192441-08-0 | SDF | Download SDF |
| PubChem ID | 3025944 | Appearance | Powder |
| Formula | C10H8BrN5OS | M.Wt | 326.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | PaTrin-2 | ||
| Solubility | DMSO : ≥ 56 mg/mL (171.69 mM) *"≥" means soluble, but saturation unknown. | ||
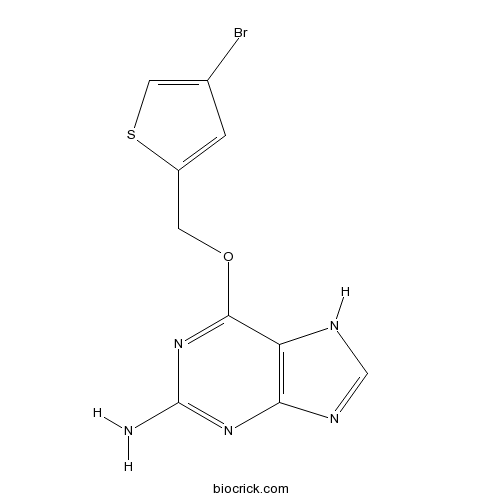
| Chemical Name | 6-[(4-bromothiophen-2-yl)methoxy]-7H-purin-2-amine | ||
| SMILES | C1=C(SC=C1Br)COC2=NC(=NC3=C2NC=N3)N | ||
| Standard InChIKey | JUJPKFNFCWJBCX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H8BrN5OS/c11-5-1-6(18-3-5)2-17-9-7-8(14-4-13-7)15-10(12)16-9/h1,3-4H,2H2,(H3,12,13,14,15,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of O6-methylguanine-DNA methyltransferase (MGMT); (IC50 = 0.009 μM in cell-free extracts from HeLa S3 cells); attenuates MGMT activity in vitro and in vivo. Enhances the antitumor activity of temozolomide in both human melanoma and MCF-7 xenografts. |

Lomeguatrib Dilution Calculator

Lomeguatrib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0659 mL | 15.3294 mL | 30.6589 mL | 61.3177 mL | 76.6471 mL |
| 5 mM | 0.6132 mL | 3.0659 mL | 6.1318 mL | 12.2635 mL | 15.3294 mL |
| 10 mM | 0.3066 mL | 1.5329 mL | 3.0659 mL | 6.1318 mL | 7.6647 mL |
| 50 mM | 0.0613 mL | 0.3066 mL | 0.6132 mL | 1.2264 mL | 1.5329 mL |
| 100 mM | 0.0307 mL | 0.1533 mL | 0.3066 mL | 0.6132 mL | 0.7665 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
The combination therapy of lomeguatrib and TMZ decreased MGMT expression, increased p53 expression and DNA fragmentation and induced apoptosis in primary GBM cell cultures and glioma cell lines without affectinf MGMT methylation and cell cycle, where the sensitivity to the therapy was associated with the structure of MGMT methylation.
Abstract
The combination of lomegutrin and TMZ decreased MGMT expression, increased p53 expression and induced apoptosis without affecting cell cycle in AA cell lines, where lomeguatrib enhanced the anti-AA activity of TMZ.
Abstract
The combination of lomeguatrib, which depletes MGMT, and dacarbazine has been assessed in a phase I trial.
Abstract
The combination of lomeguatrip and irinotecan has been assessed for MTD, safety, toxicity and clinical pharmacology in patients with metastatic cancer.
Abstract
The minimal dosage of lomeguatrib to neutralize MGMT activity for 12 hours has been determined in patients with prostate, CNS and colorectal cancer.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Lomeguatrib is an O6-methylguanine-DNA-methyl-transferase (MGMT) inhibitor [1], with an IC50 value of about 6 nM to inactivate MGMT in MCF-7 cells, effectively [2].
MGMT activity is closely related to MTIC (a metabolite of dacarbazine)-mediated DNA damage [1].
Lomeguatrib sensitized MGMT-activity-bearing A375P cells to temozolomide (TMZ), but it failed to affect the effect of dacarbazine (DTIC). In other mutBRAF cells and several mutNRAS cell lines such as WM1361, similar results were obtained [1].
With one exception, patients treated with lomeguatrib showed no active or very low MGMT in PBMCs. Lomeguatrib at a dose of 20 mg resulted in 16.7 fmol/μg DNA active MGMT in a CNS-tumor-bearing patient. This patient showed a percentage of 25% for inactive tumor MGMT. This percentage was lower than that in the other two CNS patients with lomeguatrib at the same dose. Different tumor types showed remarkable differences in total tumor MGMT. Prostate cancers had the highest (554 ± 404 fmol/mg protein), CNS tumors had the lowest (89.9 ± 44.5 fmol/mg protein), and colorectal tumors had intermediate levels of total protein (244 ± 181 fmol/mg protein). In the colorectal cancer, the primary CNS tumor, and the prostate cancer of patients, increasing lomeguatrib doses resulted in increasing inactive MGMT proportions [3].
References:
[1]. Imanol Arozarena, Ibai Goicoechea, Oihane Erice, et al. Differential chemosensitivity to antifolate drugs between RAS and BRAF melanoma cells. Molecular Cancer, 2014, 13:154.
[2]. M Clemons, J Kelly, AJ Watson, et al. O6-(4-bromothenyl)guanine reverses temozolomide resistance in human breast tumour MCF-7 cells and xenografts. British Journal of Cancer, 2005, 93:1152-1156.
[3]. Amanda J. Watson, Ami Sabharwal, Mary Thorncroft, et al. Tumor O6-methylguanine-DNA Methyltransferase Inactivation by Oral Lomeguatrib. Clinical Cancer Research, 2010, 16(2):743-9.
- Neuropeptide SF (human)
Catalog No.:BCC5829
CAS No.:192387-39-6
- Neuropeptide AF (human)
Catalog No.:BCC5854
CAS No.:192387-38-5
- Prazosin HCl
Catalog No.:BCC2505
CAS No.:19237-84-4
- CGP 71683 hydrochloride
Catalog No.:BCC7283
CAS No.:192322-50-2
- SIB 1508Y maleate
Catalog No.:BCC7975
CAS No.:192231-16-6
- Galanganone C
Catalog No.:BCN7486
CAS No.:1922129-46-1
- Galanganone B
Catalog No.:BCN7485
CAS No.:1922129-43-8
- Galanganone A
Catalog No.:BCN7484
CAS No.:1922129-42-7
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
- Prazosin
Catalog No.:BCC4081
CAS No.:19216-56-9
- Harpagoside
Catalog No.:BCN4995
CAS No.:19210-12-9
- Ergosta-4,6,8(14),22-tetraen-3-one
Catalog No.:BCN1183
CAS No.:19254-69-4
- 9alpha,11,12-Trihydroxydrim-7-en-6-one
Catalog No.:BCN7388
CAS No.:192566-65-7
- 11α-Hydroxycanrenone
Catalog No.:BCC8433
CAS No.:192569-17-8
- PPADS tetrasodium salt
Catalog No.:BCC6725
CAS No.:192575-19-2
- 9-Benzoylcarbazole
Catalog No.:BCC8799
CAS No.:19264-68-7
- H-DL-HoSer-OH
Catalog No.:BCC3242
CAS No.:1927-25-9
- PD 161570
Catalog No.:BCC7765
CAS No.:192705-80-9
- C527
Catalog No.:BCC3972
CAS No.:192718-06-2
- Lopinavir
Catalog No.:BCC3621
CAS No.:192725-17-0
- 3-Isomangostin
Catalog No.:BCN1214
CAS No.:19275-46-8
- Cudraflavone B
Catalog No.:BCN8067
CAS No.:19275-49-1
- Cyclomulberrin
Catalog No.:BCN3374
CAS No.:19275-51-5
Effect of lomeguatrib-temozolomide combination on MGMT promoter methylation and expression in primary glioblastoma tumor cells.[Pubmed:23519841]
Tumour Biol. 2013 Jun;34(3):1935-47.
Temozolomide (TMZ) is commonly used in the treatment of glioblastoma (GBM). The MGMT repair enzyme (O (6)-methylguanine-DNA methyltransferase) is an important factor causing chemotherapeutic resistance. MGMT prevents the formation of toxic effects of alkyl adducts by removing them from the DNA. Therefore, MGMT inhibition is an interesting therapeutic approach to circumvent TMZ resistance. The aim of the study was to investigate the effect of the combination of Lomeguatrib (an MGMT inactivator) with TMZ, on MGMT expression and methylation. Primary cell cultures were obtained from GBM tumor tissues. The sensitivity of primary GBM cell cultures and GBM cell lines to TMZ, and to the combination of TMZ and Lomeguatrib, was determined by a cytotoxicity assay (MTT). MGMT and p53 expression, and MGMT methylation were investigated after drug application. In addition, the proportion of apoptotic cells and DNA fragmentation was analyzed. The combination of TMZ and Lomeguatrib in primary GBM cell cultures and glioma cell lines decreased MGMT expression, increased p53 expression, and did not change MGMT methylation. Moreover, apoptosis was induced and DNA fragmentation was increased in cells. In addition, we also showed that Lomeguatrib-TMZ combination did not have any effect on the cell cycle. Finally, we determined that the sensitivity of each primary GBM cells and glioma cell lines to the Lomeguatrib-TMZ combination was different and significantly associated with the structure of MGMT methylation. Our study suggests that Lomeguatrib can be used with TMZ for GBM treatment, although further clinical studies will be needed so as to determine the feasibility of this therapeutic approach.
Inhibition of DNA repair with MGMT pseudosubstrates: phase I study of lomeguatrib in combination with dacarbazine in patients with advanced melanoma and other solid tumours.[Pubmed:21811257]
Br J Cancer. 2011 Sep 6;105(6):773-7.
BACKGROUND: The DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) reverses the O6-methylguanine (O6-meG) lesion induced by dacarbazine. Depletion of MGMT can be achieved using O6-meG pseudosubstrates. Herein, we report the first phase I experience of the novel O6-meG pseudosubstrate Lomeguatrib, combined with dacarbazine. METHODS: This is a phase I dose-escalation study to determine the maximum tolerated dose and recommended phase II dose (RP2D) of Lomeguatrib combined with a single dose of dacarbazine on a 21-day schedule. RESULTS: The vast majority of the 41 patients enrolled had metastatic melanoma (36/41) and most had no previous chemotherapy (30/41). The most frequent non-hematological adverse events (AEs) were nausea (52%), and fatigue (42%). The most frequent AEs of grade 3-4 severity were neutropaenia (42%), leukopaenia (17%), and thrombocytopaenia (12%). Only 1 patient had a partial response and 10 patients had stable disease. CONCLUSION: The RP2D of Lomeguatrib was 40 mg orally twice daily for 10 days combined with 400 mg m(-2) of dacarbazine IV on day 2. Oral administration of Lomeguatrib substantially increases the haematological toxicity of dacarbazine consistent with experience with other O6-meG pseudosubstrates.
Chemotherapeutic resistance in anaplastic astrocytoma cell lines treated with a temozolomide-lomeguatrib combination.[Pubmed:24368590]
Mol Biol Rep. 2014 Feb;41(2):697-703.
The treatment of anaplastic astrocytoma (AA) is controversial. New chemotherapeutic approaches are needed for AA treatment. Temozolomide (TMZ) is one of the chemotherapeutic drugs for the treatment of AA. The cytotoxic effects of TMZ can be removed by the MGMT (O(6)-methylguanine-DNA methyltransferase) enzyme. Then, chemotherapeutic resistance to TMZ occurs. MGMT inhibition by MGMT inactivators (such as Lomeguatrib) is an important anticancer therapeutic approach to circumvent TMZ resistance. We aim to investigate the effect of TMZ-Lomeguatrib combination on MGMT expression and TMZ sensitivity of SW1783 and GOS-3 AA cell lines. The sensitivity of SW1783 and GOS-3 cell lines to TMZ and to the combination of TMZ and Lomeguatrib was determined by a cytotoxicity assay. MGMT methylation was detected by MS-PCR. MGMT and p53 expression were investigated by real-time PCR after drug treatment, and the proportion of apoptotic cells was analyzed by flow cytometry. When the combination of TMZ-Lomeguatrib (50 muM) was used in AA cell lines, IC50 values were reduced compared to only using TMZ. MGMT expression was decreased, p53 expression was increased, and the proportion of apoptotic cells was induced in both cell lines. The Lomeguatrib-TMZ combination did not have any effect on the cell cycle and caused apoptosis by increasing p53 expression and decreasing MGMT expression. Our study is a pilot study investigating a new therapeutic approach for AA treatment, but further research is needed.
Tumor O(6)-methylguanine-DNA methyltransferase inactivation by oral lomeguatrib.[Pubmed:20068091]
Clin Cancer Res. 2010 Jan 15;16(2):743-9.
PURPOSE: A major mechanism of resistance to chlorethylnitrosureas and methylating agents involves the DNA repair protein O(6)-methylguanine-DNA methyltransferase (MGMT). We sought to determine the dose of oral 6-(4-bromo-2-thienyl) methoxy purin-2-amine (Lomeguatrib), a pseudosubstrate inactivator of MGMT, required to render active protein undetectable 12 hours after dosing in prostate, primary central nervous system (CNS), and colorectal cancer patients. EXPERIMENTAL DESIGN: Lomeguatrib was administered orally as a single dose (20-160 mg) approximately 12 hours before tumor resection. Dose escalation was projected to continue until grade 2 toxicity or until complete inactivation of tumor MGMT was encountered. Total MGMT protein levels were quantified by ELISA, and active protein levels were quantified by biochemical assay. MGMT promoter methylation was determined in glioblastoma DNA by methylation-specific PCR. RESULTS: Thirty-seven patients were dosed with Lomeguatrib, and 32 informative tumor samples were obtained. Mean total MGMT level varied between tumor types: 554 +/- 404 fmol/mg protein (+/-SD) for prostate cancer, 87.4 +/- 40.3 fmol/mg protein for CNS tumors, and 244 +/- 181 fmol/mg protein for colorectal cancer. MGMT promoter hypermethylation did not correlate with total protein expression. Consistent total MGMT inactivation required 120 mg of Lomeguatrib in prostate and colorectal cancers. Complete consistent inactivation in CNS tumors was observed only at the highest dose of Lomeguatrib (160 mg). CONCLUSIONS: Total MGMT inactivation can be achieved in prostate, primary CNS, and colorectal cancers with a single administration of 120 or 160 mg Lomeguatrib. The dose needed did not correlate with mean total MGMT protein concentrations. One hundred twenty to 160 mg/d of Lomeguatrib should be administered to achieve total MGMT inactivation in future studies.
O6-(4-bromothenyl)guanine reverses temozolomide resistance in human breast tumour MCF-7 cells and xenografts.[Pubmed:16278661]
Br J Cancer. 2005 Nov 14;93(10):1152-6.
Tumour resistance to chemotherapy involving methylating agents such as DTIC (dacarbazine) and temozolomide is linked to expression of the DNA repair protein O(6)-alkylguanine-DNA alkyltransferase (MGMT). There is considerable interest in improving the efficacy of such O(6)-alkylating chemotherapy by the prior inactivation of MGMT. We have examined the effect of the modified guanine base, O(6)-(4-bromothenyl)guanine (PaTrin-2, Patrin, Lomeguatrib) on MGMT activity and cell or xenograft tumour growth inhibition by temozolomide in the human breast carcinosarcoma cell line, MCF-7. PaTrin-2 effectively inactivated MGMT in MCF-7 cells (IC(50) approximately 6 nM) and in xenografts there was complete inactivation of MGMT within 2 h of dosing (20 mg kg(-1) i.p.) and only slight recovery by 24 h. MGMT inactivation in a range of murine host tissues varied between complete and approximately 60%, with extensive recovery by 24 h. PaTrin-2 (10 microM) substantially increased the growth inhibitory effects of temozolomide in MCF-7 cells (D(60)=10 microM with PaTrin-2 vs 400 microM without). In MCF-7 xenografts, neither temozolomide (100 mg kg(-1) day(-1) for 5 days) nor PaTrin-2 (20 mg kg(-1) day(-1) for 5 days) had any significant effect on tumour growth. In contrast, the PaTrin-2-temozolomide combination produced a substantial tumour growth delay: median tumour quintupling time was increase by 22 days (P<0.005) without any significant increase in toxicity as assessed from animal weight. A PaTrin-2-temozolomide combination may therefore be beneficial in the treatment of human breast cancers.
Sensitization of a human ovarian cancer cell line to temozolomide by simultaneous attenuation of the Bcl-2 antiapoptotic protein and DNA repair by O6-alkylguanine-DNA alkyltransferase.[Pubmed:15486188]
Mol Cancer Ther. 2004 Oct;3(10):1215-20.
Temozolomide is an alkylating agent that mediates its cytotoxic effects via O(6)-methylguanine (O(6)-meG) adducts in DNA. O(6)-alkylguanine-DNA-alkyltransferase (MGMT) can repair such adducts and therefore constitutes a major resistance mechanism to the drug. MGMT activity can be attenuated in vitro and in vivo by the pseudosubstrate O(6)-(4-bromothenyl)guanine (PaTrin-2, Patrin, Lomeguatrib), which in clinical trials is in combination with temozolomide. Resistance to cytotoxic agents can also be mediated by the Bcl-2 protein, which inhibits apoptosis and is frequently up-regulated in tumor cells. Attenuation of Bcl-2 expression can be affected by treatment of cells with the antisense oligonucleotide, oblimersen sodium (Genasense), currently in phase III clinical trials in combination with the methylating agent dacarbazine. Using a human ovarian cancer cell line (A2780) that expresses both Bcl-2 and MGMT, we show that cells treated with active dose levels of either oblimersen (but not control reverse sequence or mismatch oligonucleotides) or PaTrin-2 are substantially sensitized to temozolomide. Furthermore, the exposure of oblimersen-pretreated cells to PaTrin-2 leads to an even greater sensitization of these cells to temozolomide. Thus, growth of cells treated only with temozolomide (5 microg/mL) was 91% of control growth, whereas additional exposure to PaTrin-2 alone (10 micromol/L) or oblimersen alone (33 nmol/L) reduced this to 81% and 66%, respectively, and the combination of PaTrin-2 (10 micromol/L) and oblimersen (33 nmol/L) reduced growth to 25% of control. These results suggest that targeting both Bcl-2 with oblimersen and MGMT with PaTrin-2 would markedly enhance the antitumor activity of temozolomide and merits testing in clinical trials.
Monosaccharide-linked inhibitors of O(6)-methylguanine-DNA methyltransferase (MGMT): synthesis, molecular modeling, and structure-activity relationships.[Pubmed:11708909]
J Med Chem. 2001 Nov 22;44(24):4050-61.
A series of potential inhibitors of the human DNA repair protein O(6)-methylguanine-DNA methyltransferase (MGMT) were synthesized, characterized in detail by NMR, and tested for their ability to deplete MGMT activity in vitro. The new compounds, omega-[O(6)-R-guan-9-yl]-(CH(2))(n)-beta-d-glucosides with R = benzyl or 4-bromothenyl and omega = n = 2, 4,. 12, were compared with the established inhibitors O(6)-benzylguanine (O(6)-BG), 8-aza-O(6)-benzylguanine (8-aza-BG), and O(6)-(4-bromothenyl)guanine (4-BTG), which exhibit in an in vitro assay IC(50) values of 0.62, 0.038, and 0.009 microM, respectively. Potential advantages of the glucosides are improved water solubility and selective uptake in tumor cells. The 4-BTG glucosides with n = 2, 4, 6 show moderate inhibition with an IC(50) of ca. 0.5 microM, while glucosides derived from BG and 8-aza-BG showed significantly poorer inhibition compared to the parent compounds. The 4-BTG glucosides with n = 8, 10, 12 were effective inhibitors with IC(50) values of ca. 0.03 microM. To understand this behavior, extensive molecular modeling studies were performed using the published crystal structure of MGMT (PDB entry: ). The inhibitor molecules were docked into the BG binding pocket, and molecular dynamics simulations with explicit water molecules were carried out. Stabilization energies for the interactions of specific regions of the inhibitor and individual amino acid residues were calculated. The alkyl spacer is located in a cleft along helix 6 of MGMT. With increasing spacer length there is increasing interaction with several amino acid residues which play an important role in the proposed nucleotide flipping mechanism required for DNA repair.


