LimocitrinCAS# 489-33-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 489-33-8 | SDF | Download SDF |
| PubChem ID | 5489485 | Appearance | Yellow powder |
| Formula | C17H14O8 | M.Wt | 346.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Gossypetin 3',8-dimethyl ether; 8-Methoxyisorhamnetin; Sedoflorigenin; 3,4',5,7-Tetrahydroxy 3',8-dimethoxyflavone | ||
| Solubility | Soluble in acetone, chloroform and DMSO | ||
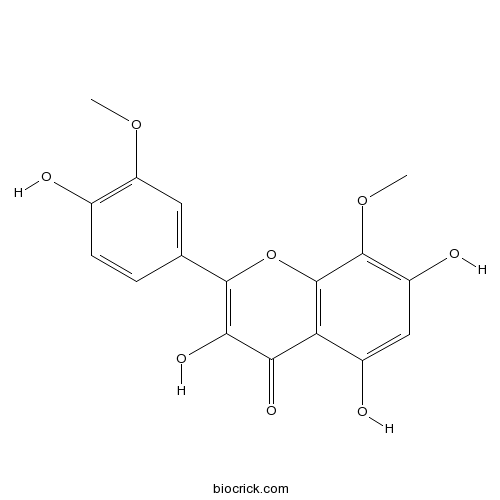
| Chemical Name | 3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-8-methoxychromen-4-one | ||
| SMILES | COC1=C(C=CC(=C1)C2=C(C(=O)C3=C(O2)C(=C(C=C3O)O)OC)O)O | ||
| Standard InChIKey | IBXCKSUZOFKGSB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O8/c1-23-11-5-7(3-4-8(11)18)15-14(22)13(21)12-9(19)6-10(20)16(24-2)17(12)25-15/h3-6,18-20,22H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Limocitrin is a constituent of citrus fruit peels, belongs to the family of Flavonols. |

Limocitrin Dilution Calculator

Limocitrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8877 mL | 14.4383 mL | 28.8767 mL | 57.7534 mL | 72.1917 mL |
| 5 mM | 0.5775 mL | 2.8877 mL | 5.7753 mL | 11.5507 mL | 14.4383 mL |
| 10 mM | 0.2888 mL | 1.4438 mL | 2.8877 mL | 5.7753 mL | 7.2192 mL |
| 50 mM | 0.0578 mL | 0.2888 mL | 0.5775 mL | 1.1551 mL | 1.4438 mL |
| 100 mM | 0.0289 mL | 0.1444 mL | 0.2888 mL | 0.5775 mL | 0.7219 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Icariin
Catalog No.:BCN6311
CAS No.:489-32-7
- Elesclomol (STA-4783)
Catalog No.:BCC2337
CAS No.:488832-69-5
- N-Acetylstepharine
Catalog No.:BCN7566
CAS No.:4880-87-9
- Furan-3-carboxylic acid
Catalog No.:BCN6397
CAS No.:488-93-7
- D-arabinitol
Catalog No.:BCN5595
CAS No.:488-82-4
- D-Ribitol-5-phosphate
Catalog No.:BCC4838
CAS No.:35320-17-3
- vibo-Quercitol
Catalog No.:BCN5594
CAS No.:488-76-6
- Allitol
Catalog No.:BCN5593
CAS No.:488-44-8
- 3-Methylcatechol
Catalog No.:BCN3925
CAS No.:488-17-5
- Thesinine
Catalog No.:BCN1990
CAS No.:488-02-8
- Heleurine
Catalog No.:BCN1953
CAS No.:488-00-6
- Curcumol
Catalog No.:BCN5976
CAS No.:4871-97-0
- Gossypetin
Catalog No.:BCN8075
CAS No.:489-35-0
- Globulol
Catalog No.:BCN6901
CAS No.:489-41-8
- Guaiazulen
Catalog No.:BCC8180
CAS No.:489-84-9
- Guaiol
Catalog No.:BCN6619
CAS No.:489-86-1
- ML 213
Catalog No.:BCC6213
CAS No.:489402-47-3
- SBC-115076
Catalog No.:BCC6440
CAS No.:489415-96-5
- a-Truxilline
Catalog No.:BCN1947
CAS No.:490-17-5
- Beta-Tocotrienol
Catalog No.:BCN3725
CAS No.:490-23-3
- Robinetin
Catalog No.:BCN5596
CAS No.:490-31-3
- Epicatechin
Catalog No.:BCN5597
CAS No.:490-46-0
- Gaultherin
Catalog No.:BCN2482
CAS No.:490-67-5
- Homoquinolinic acid
Catalog No.:BCC6570
CAS No.:490-75-5
Fractionation of orange peel phenols in ultrafiltered molasses and mass balance studies of their antioxidant levels.[Pubmed:15675808]
J Agric Food Chem. 2004 Dec 15;52(25):7586-92.
Orange peel molasses, a byproduct of juice production, contains high concentrations of phenols, including numerous flavanone and flavone glycosides, polymethoxylated flavones, hydroxycinnamates, and other miscellaneous phenolic glycosides and amines. Extensive fractionation of these phenols was achieved by adsorption, ion exchange, and size exclusion chromatography. Size exclusion chromatography effectively separated the different classes of flavonoids in ultrafiltered molasses, including the polymethoxylated flavones, flavanone-O-trisaccharides, flavanone- and flavone-O-disaccharides, and, finally, flavone-C-glycosides. Mass spectral analysis of the early-eluting flavonoid fractions off the size exclusion column revealed a broad collection of minor-occurring flavone glycosides, which included, in part, glycosides of Limocitrin, limocitrol, and chrysoeriol. Most hydroxycinnamates in the molasses were recovered by ion exchange chromatography, which also facilitated the recovery of fractions containing many other miscellaneous phenols. Total antioxidant levels and total phenolic contents were measured for the separate categories of phenols in the molasses. Inhibition of the superoxide anion reduction of nitroblue tetrazolium showed that a significant amount of the total antioxidant activity in orange peel molasses was attributable to minor-occurring flavones. The miscellaneous phenolic-containing fractions, in which a large portion of the total phenolic content in molasses occurred, also constituted a major portion of the total antioxidants in ultrafiltered molasses.
New validated high-performance liquid chromatographic method for simultaneous analysis of ten flavonoid aglycones in plant extracts using a C18 fused-core column and acetonitrile-tetrahydrofuran gradient.[Pubmed:22807401]
J Sep Sci. 2012 Sep;35(17):2174-83.
An HPLC method of high resolution has been developed and validated for the simultaneous determination of ten prominent flavonoid aglycones in plant materials using a fused-core C18-silica column (Ascentis(R) Express, 4.6 mm x 150 mm, 2.7 mum). The separation was accomplished with an acetonitrile-tetrahydrofuran gradient elution at a flow rate of 1 mL/min and temperature of 30 degrees C. UV spectrophotometric detection was employed at 370 nm for flavonols (quercetin [QU], myricetin [MY], isorhamnetin [IS], kaempferol [KA], sexangularetin [SX], and Limocitrin [LM]) and 340 nm for flavones (apigenin [AP], acacetin [AC], chrysoeriol [CH], and luteolin [LU]). The high resolution of critical pairs QU/LU (10.50), QU/CH (3.40), AP/CH (2.51), SX/LM (2.30), and IS/KA (2.70) was achieved within 30.3 min. The observed column back pressure was less than 4300 psi, thus acceptable for conventional HPLC equipment. The method was sensitive enough having LODs of 0.115-0.525 ng and good linearity (r > 0.9999) over the test range. The precision values, expressed as RSD values, were <7.5%, and the accuracy was in the range of 95.3-100.2% for all analytes except MY (73.8%). The method was successfully employed for the determination of flavonoids in several medicinal plants, such as Ginkgo biloba, Betula pendula, and a variety of Sorbus species.


