LaxogeninCAS# 1177-71-5 |
Quality Control & MSDS
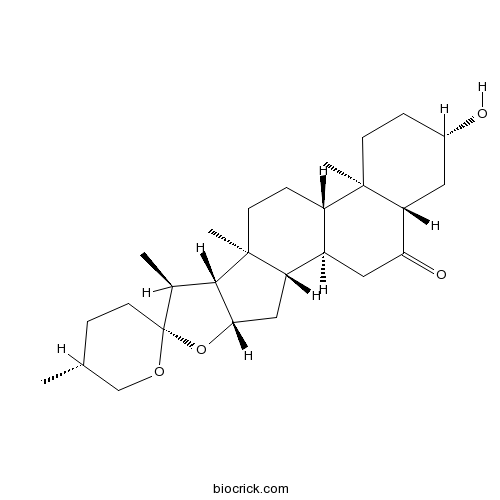
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1177-71-5 | SDF | Download SDF |
| PubChem ID | 10950057 | Appearance | White crystalline powder |
| Formula | C27H42O4 | M.Wt | 430.62 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Laxogenine | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2S,4S,5'R,6R,7S,8R,9S,12S,13R,16S,18S)-16-hydroxy-5',7,9,13-tetramethylspiro[5-oxapentacyclo[10.8.0.02,9.04,8.013,18]icosane-6,2'-oxane]-19-one | ||
| SMILES | CC1CCC2(C(C3C(O2)CC4C3(CCC5C4CC(=O)C6C5(CCC(C6)O)C)C)C)OC1 | ||
| Standard InChIKey | WOJKRRDDERNLBU-BOYSPROGSA-N | ||
| Standard InChI | InChI=1S/C27H42O4/c1-15-5-10-27(30-14-15)16(2)24-23(31-27)13-20-18-12-22(29)21-11-17(28)6-8-25(21,3)19(18)7-9-26(20,24)4/h15-21,23-24,28H,5-14H2,1-4H3/t15-,16+,17+,18-,19+,20+,21-,23+,24+,25-,26+,27-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Laxogenin Dilution Calculator

Laxogenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3222 mL | 11.6112 mL | 23.2223 mL | 46.4447 mL | 58.0558 mL |
| 5 mM | 0.4644 mL | 2.3222 mL | 4.6445 mL | 9.2889 mL | 11.6112 mL |
| 10 mM | 0.2322 mL | 1.1611 mL | 2.3222 mL | 4.6445 mL | 5.8056 mL |
| 50 mM | 0.0464 mL | 0.2322 mL | 0.4644 mL | 0.9289 mL | 1.1611 mL |
| 100 mM | 0.0232 mL | 0.1161 mL | 0.2322 mL | 0.4644 mL | 0.5806 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- DL-Syringaresinol
Catalog No.:BCN6053
CAS No.:1177-14-6
- LY 255283
Catalog No.:BCC7290
CAS No.:117690-79-6
- Agomelatine hydrochloride
Catalog No.:BCC4210
CAS No.:1176316-99-6
- 3-Cyano-7-ethoxycoumarin
Catalog No.:BCC7979
CAS No.:117620-77-6
- 16-Oxocleroda-3,13E-dien-15-oic acid
Catalog No.:BCN7286
CAS No.:117620-72-1
- 1-Hydroxy-1-(4-hydroxy-2-methoxyphenyl)-3-(4-hydroxyphenyl)propan-2-one
Catalog No.:BCN1607
CAS No.:117614-84-3
- 6-Hydroxymethylherniarin
Catalog No.:BCN3573
CAS No.:117597-79-2
- Coronarin E
Catalog No.:BCN6052
CAS No.:117591-81-8
- Calpeptin
Catalog No.:BCC2351
CAS No.:117591-20-5
- DMXAA (Vadimezan)
Catalog No.:BCC3644
CAS No.:117570-53-3
- threo-6'-Hydroxyustusolate C
Catalog No.:BCN6930
CAS No.:1175543-07-3
- Ustusolate E
Catalog No.:BCN7789
CAS No.:1175543-06-2
- Dexamethasone acetate
Catalog No.:BCC4775
CAS No.:1177-87-3
- Doramectin
Catalog No.:BCC1536
CAS No.:117704-25-3
- CKI 7 dihydrochloride
Catalog No.:BCC5614
CAS No.:1177141-67-1
- (R)-(+)-Blebbistatin
Catalog No.:BCC7195
CAS No.:1177356-70-5
- Felbamate hydrate
Catalog No.:BCC4160
CAS No.:1177501-39-1
- Forsythoside I
Catalog No.:BCN6430
CAS No.:1177581-50-8
- N20C hydrochloride
Catalog No.:BCC7292
CAS No.:1177583-87-7
- SMANT hydrochloride
Catalog No.:BCC6254
CAS No.:1177600-74-6
- CGH 2466 dihydrochloride
Catalog No.:BCC7338
CAS No.:1177618-54-0
- Azithromycin Dihydrate
Catalog No.:BCC4631
CAS No.:117772-70-0
- Decumbenine C
Catalog No.:BCC8314
CAS No.:117772-89-1
- Desmethyl-YM 298198
Catalog No.:BCC7365
CAS No.:1177767-57-5
The power of hyphenated chromatography-Time of flight mass spectrometry for unequivocal identification of spirostanes in bodybuilding dietary supplements.[Pubmed:30753977]
J Pharm Biomed Anal. 2019 Apr 15;167:74-82.
A previously unidentified purported botanical ingredient was found in dietary supplements marketed for anabolic benefits. In an attempt to assess the 'naturalness' of a group of steroid-like compounds called Laxogenins, a UHPLC-QToF method was developed. Several dietary supplements claim to contain 5alpha-hydroxy Laxogenin, which is a derivative of a naturally occurring spirostane-type steroid, Laxogenin. Although Laxogenin has been isolated from the rhizomes of Smilax sieboldii, 5alpha-hydroxy Laxogenin has not been isolated or reported from any natural source. These derivatives of Laxogenins have untested anabolic properties. Due to the low UV absorbance of the spirostanes, a mass spectrometric method in positive ion mode was developed for unambiguous identification of Laxogenin and closely related compounds. To show the utility of the developed method, twelve dietary supplements labeled to contain 5alpha-hydroxy Laxogenin or Laxogenin as 5alpha-hydroxy Laxogenin were analyzed as a proof-of-concept. Five supplements did not contain any 5alpha-hydroxy Laxogenin, whereas in the remaining seven samples, spirostane-type contaminants were identified along with the labeled 5alpha-hydroxy Laxogenin. The identity of some of these contaminants was established based on reference standards along with mass fragmentation patterns. One of the unlabeled contaminants was identified as the phytosteroid saponin, diosgenin, a common starting precursor of several steroidal drugs. Several synthetic derivatives of diosgenin were identified in the eight products. These findings indicate that the labeled 5alpha-hydroxy Laxogenin along with other spirostanes found in supplements are synthetic and signify a lack of quality controls. Additionally, an unlabeled, anabolic androgenic steroid, arimistane, an aromatase inhibitor, was also identified in one product. Laxogenin, was not detected in any of the samples analyzed during this investigation.
Synthesis of brassinosteroids analogues from laxogenin and their plant growth promotion.[Pubmed:25311045]
Nat Prod Res. 2015;29(2):149-57.
Four steroid saponins (2-5) and three derivatives (6-8) were synthesised from Laxogenin. Four of them were new compounds: (25R)-3beta-(2,3,4,6-tetra-O-acetyl-beta-D-galactopyranosyloxy)-5alpha-spirostan- 6-one (3), (25R)-3beta-(beta-D-galactopyranosyloxy)-5alpha-spirostan-6-one (5), 3beta,16-diacetyl-26-hydroxy-5alpha-cholestan-6,22-dione (6) and 16-acetyl-3beta,26-dihydroxy-5alpha-cholestan-6,22-dione (7). All the compounds showed plant growth-promoting activity in the radish hypocotyl elongation and cotyledon expansion bioassay. Above all, 2 and 6 were found to be more active.
Structure and cytotoxicity of steroidal glycosides from Allium schoenoprasum.[Pubmed:23357597]
Phytochemistry. 2013 Apr;88:61-6.
A phytochemical analysis of the whole plant of Allium schoenoprasum, has led to the isolation of four spirostane-type glycosides (1-4), and four known steroidal saponins. Their structures were elucidated mainly by 2D NMR spectroscopic analysis and mass spectrometry as (20S,25S)-spirost-5-en-3beta,12beta,21-triol 3-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranoside (1), (20S,25S)-spirost-5-en-3beta,11alpha,21-triol 3-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranoside (2), Laxogenin 3-O-alpha-L-rhamnopyranosyl-(1-->2)-[beta-D-glucopyranosyl-(1-->4)]-beta-D-glucop yranoside (3), and (25R)-5alpha-spirostan-3beta,11alpha-diol 3-O-beta-D-glucopyranosyl-(1-->3)-[beta-D-glucopyranosyl-(1-->4)]-beta-D-galactop yranoside (4). Four of the isolated compounds were tested for cytotoxic activity against the HCT 116 and HT-29 human colon cancer cell lines.
New insights into the structure-cytotoxicity relationship of spirostan saponins and related glycosides.[Pubmed:22405922]
Bioorg Med Chem. 2012 Apr 15;20(8):2690-700.
A variety of spirostan saponins and related glycosides were synthesized and evaluated for their cytotoxicity against the human myeloid leukemia cell line (HL-60). A linear glycosylation strategy allowed for accessing a variety of functionalization patterns at both the spirostanic and the saccharide moieties, which provides new information regarding the structure-cytotoxicity relationship of this family of steroidal glycosides. Intriguing results were achieved with respect to hecogenyl and 5alpha-hydroxy-laxogenyl beta-chacotriosides, turning out to be the former very cytotoxic and the latter no cytotoxic at all. Importantly, the partially pivaloylated beta-d-glucosides of 5alpha-hydroxy-Laxogenin were the most potent cytotoxic compounds among all tested glycosides. This comprises the first report on acylated spirostanyl glucosides displaying significant cytotoxicity, and therefore, it opens up new opportunities toward the development of saponin analogues as anticancer agents.
Glycosyl trifluoroacetimidates. 2. Synthesis of dioscin and xiebai saponin I.[Pubmed:12467439]
J Org Chem. 2002 Dec 13;67(25):9099-102.
Two trisaccharide steroidal saponins, dioscin (1) and Xiebai saponin I (2) with various bioactivities, were efficiently synthesized using the newly developed glycosyl N-phenyl trifluoroacetimidates (10-13) as glycosylation donors. Thus, dioscin was synthesized in five steps and a 33% overall yield from diosgenin and glycosyl trifluoroacetimidates (10 and 11). Xiebai saponin I was synthesized in eight steps and a 32% overall yield from Laxogenin and glycosyl trifluoroacetimidates (10, 12, and 13), whereupon, the rare steroid Laxogenin was prepared from diosgenin in four steps and an overall 69% yield. All the glycosylation reactions involved in the present syntheses demonstrated that glycosyl trifluoroacetimidates were successful donors comparable to the corresponding glycosyl trichloroacetimidates.
Saponins isolated from Allium chinense G. Don and antitumor-promoting activities of isoliquiritigenin and laxogenin from the same drug.[Pubmed:10823685]
Biol Pharm Bull. 2000 May;23(5):660-2.
Investigation of the Chinese crude drug "Xiebai," the bulbs of Allium chinense G. Don (Liliaceae), led to the isolation of 2 saponins, xiebai-saponin I (Laxogenin 3-O-beta-xylopyranosyl (1-->4)-[alpha-arabinopyranosyl (1-->6)-beta-glucopyranoside) (1) and Laxogenin 3-O-alpha-arabinopyranosyl (1-->6)-beta-glucopyranoside (2), and the aglycone, Laxogenin (3), together with 2 chalcones, isoliquiritigenin (4) and isoliquiritigenin-4-O-glucoside (5), and beta-sitosterol glucoside (6). Compounds 1-5 were tested in vitro for their inhibitory effect on the 12-O-tetradecanoylphorbol-13-acetate (TPA)-stimulated 32Pi-incorporation into phospholipids of HeLa cells. In addition to this, Laxogenin (3) was proven to have an antitumor-promoting activity in a two-stage lung carcinogenesis experiment.
Screening of anti-hypoxia/reoxygenation agents by an in vitro method. Part 2: Inhibition of tyrosine kinase activation prevented hypoxia/reoxygenation-induced injury in endothelial gap junctional intercellular communication.[Pubmed:10763583]
Planta Med. 2000 Mar;66(2):119-23.
In this study, we demonstrated that hypoxia/reoxygenation (H/R) induced an injury in gap junctional intercellular communication (GJIC) after 2 h of reoxygenation in cultured HUVEC. Free radical scavenger (DMSO) and antioxidant (SOD) did not prevent this GJIC injury at all. Protein kinase C inhibitor (calphostin C) partly blocked this injury. However, the protein tyrosine kinase (PTK) inhibitor genistein completely inhibited this GJIC injury. Compounds 1 [Laxogenin-3-O-alpha-L-arabinosyl-(1-->6)- beta-D-glucopyranoside], 2 (macrostemososide A), 3 [Laxogenin-3-O-beta-D-xylopyranosyl-(1-->4)-alpha- L-arabinopyranosyl-(1-->6)-beta-D-glucopyranoside], 4 (chinenoside II), 5 (beta-sitosterol), 6 (daucosterine), 7 (ginsenoside-Rd), 29 (isocumarine), 52 (icariin), 53 (icariside), and 54 (icaritin), which showed obvious influence on H/R-induced PTK activation as stated in Part 1 (except 1), were explored for their effects on GJIC. The results showed that compounds 2-7 and 52-57 partly protected H/R-induced GJIC injury. Compounds 5 and 6 (especially 5), which showed the strongest inhibitory effects on PTK activation, completely blocked H/R-provoked GJIC injury. Compound 1, which did not influence PTK activation, failed to prevent this GJIC injury. In contrast, compound 29, which significantly promoted PTK activation, enhanced this H/R-induced GJIC injury further. Western blotting of connexin 43, an important gap junctional protein for modulating GJIC in HUVEC, revealed that interference with the gap junctional protein might be the most direct mechanism for compounds 2, 5, 29, and 53 to affect H/R-injured GJIC.
Screening of anti-hypoxia/reoxygenation agents by an in vitro model. Part 1: Natural inhibitors for protein tyrosine kinase activated by hypoxia/reoxygenation in cultured human umbilical vein endothelial cells.[Pubmed:10763582]
Planta Med. 2000 Mar;66(2):114-8.
Protein tyrosine kinase (PTK) signaling pathways play important roles in ischemia/reperfusion (I/R) or hypoxia/reoxygenation (H/R) injuries. Inhibition of PTK activation can protect against I/R- or H/R-induced damages. As one part of our work for seeking bioactive compounds from natural sources against I/R or H/R, in the present study we examined the effects of 54 compounds purified from various traditional Chinese herbs on H/R-induced PTK activation by means of an in vitro H/R model in cultured human umbilical vein endothelial cells (HUVEC). The results demonstrated that an increase in PTK activation was induced after 2 h of reoxygenation. Compounds 2 (macrostemososide A), 3 (Laxogenin-3-O-beta-D-xylopyranosyl-(1-->4)-alpha-L-arabinopyra nosyl- (1-->6)-beta-D-glucopyranoside), 4 (chinenoside II), 7 (ginsenoside-Rd), 52 (icariin), 53 (icariside), and 54 (icaritin) showed relatively obvious inhibition on this H/R-induced PTK activation. Compounds 5 (beta-sitosterol) and 6 (daucosterine), especially 5, completely blocked such an increased activation of PTK induced by H/R. On the contrary, compound 29 (isocumarine) significantly promoted PTK activation further. Moreover, the effects of these compounds on PTK activation were dose-dependent.
Steroidal saponins from the rhizomes of Smilax sieboldii.[Pubmed:1369386]
Phytochemistry. 1992 Jul;31(7):2445-50.
Six new steroidal saponins were isolated from the rhizomes of Smilax sieboldii. Their structures were determined by spectroscopic analysis and hydrolysis to be 3 beta-hydroxy-(25R)-5 alpha-spirostan-6-one (Laxogenin) 3-O-beta-D-glucopyranosyl-(1----4)-O-[alpha-L-arabinopyranosyl-(1- ---6)]-beta-D-glucopyranoside, Laxogenin 3-O-alpha-L-arabinopyranosyl-(1----6)-beta-D-glucopyranoside, 3 beta,27-dihydroxy-(25S)-5 alpha-spirostan-6-one 3-O-beta-D-glucopyranosyl-(1----4)-O-[alpha-L-arabinopyranosyl-(1- ---6)]- beta-D-glucopyranoside, 26-O-beta-D-glucopyranosyl-3 beta,22 xi,26-trihydroxy-(25R)-5 alpha-furostan-6-one 3-O-alpha-L-arabinopyranosyl-(1----6)-beta-D-glucopyranoside, 26-O-beta-D-glucopyranosyl-3 beta,22 xi,26-trihydroxy-(25R)-5 alpha-furostan-6- one 3-O-beta-D-glucopyranosyl-(1----4)-O-[alpha-L-arabinopyranosyl-(1- ---6)]- beta-D-glucopyranoside and (25R)-5 alpha-spirostan-3 beta-ol (tigogenin) 3-O-beta-D-glucopyranosyl-(1----4)-O-[alpha-L-arabinopyranosyl- (1----6)]-beta-D-glucopyranoside. The inhibition of cAMP phosphodiesterase by the saponins was evaluated.


