Coronarin ECAS# 117591-81-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117591-81-8 | SDF | Download SDF |
| PubChem ID | 9971144 | Appearance | Powder |
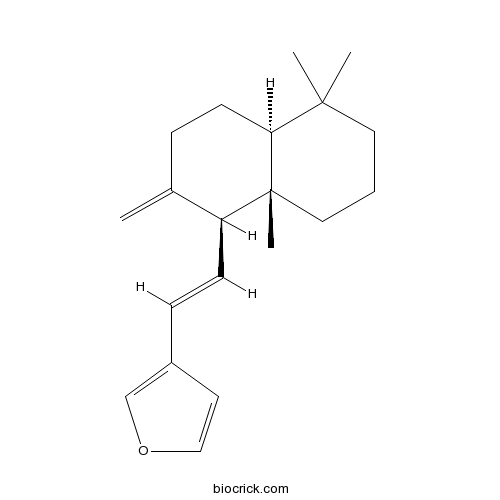
| Formula | C20H28O | M.Wt | 284.4 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-[(E)-2-[(1S,4aS,8aS)-5,5,8a-trimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]ethenyl]furan | ||
| SMILES | CC1(CCCC2(C1CCC(=C)C2C=CC3=COC=C3)C)C | ||
| Standard InChIKey | QXVXYNOIXUIXBI-NDLVVHCESA-N | ||
| Standard InChI | InChI=1S/C20H28O/c1-15-6-9-18-19(2,3)11-5-12-20(18,4)17(15)8-7-16-10-13-21-14-16/h7-8,10,13-14,17-18H,1,5-6,9,11-12H2,2-4H3/b8-7+/t17-,18-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Coronarin E exhibits weak antimicrobial activity. |
| Targets | Antifection |

Coronarin E Dilution Calculator

Coronarin E Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5162 mL | 17.5809 mL | 35.1617 mL | 70.3235 mL | 87.9044 mL |
| 5 mM | 0.7032 mL | 3.5162 mL | 7.0323 mL | 14.0647 mL | 17.5809 mL |
| 10 mM | 0.3516 mL | 1.7581 mL | 3.5162 mL | 7.0323 mL | 8.7904 mL |
| 50 mM | 0.0703 mL | 0.3516 mL | 0.7032 mL | 1.4065 mL | 1.7581 mL |
| 100 mM | 0.0352 mL | 0.1758 mL | 0.3516 mL | 0.7032 mL | 0.879 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Calpeptin
Catalog No.:BCC2351
CAS No.:117591-20-5
- DMXAA (Vadimezan)
Catalog No.:BCC3644
CAS No.:117570-53-3
- threo-6'-Hydroxyustusolate C
Catalog No.:BCN6930
CAS No.:1175543-07-3
- Ustusolate E
Catalog No.:BCN7789
CAS No.:1175543-06-2
- 2alpha,9alpha,11-Trihydroxy-6-oxodrim-7-ene
Catalog No.:BCN7741
CAS No.:1175543-03-9
- Ustusol A
Catalog No.:BCN7719
CAS No.:1175543-02-8
- Neuromedin U (rat)
Catalog No.:BCC5847
CAS No.:117505-80-3
- ROX NHS ester, pure 6- isomer
Catalog No.:BCC3587
CAS No.:117491-83-5
- Sesamoside
Catalog No.:BCN6051
CAS No.:117479-87-5
- Prionitin
Catalog No.:BCN4855
CAS No.:117469-56-4
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- BCECF-AM
Catalog No.:BCC5969
CAS No.:117464-70-7
- 6-Hydroxymethylherniarin
Catalog No.:BCN3573
CAS No.:117597-79-2
- 1-Hydroxy-1-(4-hydroxy-2-methoxyphenyl)-3-(4-hydroxyphenyl)propan-2-one
Catalog No.:BCN1607
CAS No.:117614-84-3
- 16-Oxocleroda-3,13E-dien-15-oic acid
Catalog No.:BCN7286
CAS No.:117620-72-1
- 3-Cyano-7-ethoxycoumarin
Catalog No.:BCC7979
CAS No.:117620-77-6
- Agomelatine hydrochloride
Catalog No.:BCC4210
CAS No.:1176316-99-6
- LY 255283
Catalog No.:BCC7290
CAS No.:117690-79-6
- DL-Syringaresinol
Catalog No.:BCN6053
CAS No.:1177-14-6
- Laxogenin
Catalog No.:BCN8434
CAS No.:1177-71-5
- Dexamethasone acetate
Catalog No.:BCC4775
CAS No.:1177-87-3
- Doramectin
Catalog No.:BCC1536
CAS No.:117704-25-3
- CKI 7 dihydrochloride
Catalog No.:BCC5614
CAS No.:1177141-67-1
- (R)-(+)-Blebbistatin
Catalog No.:BCC7195
CAS No.:1177356-70-5
Concise syntheses of coronarin A, coronarin E, austrochaparol and pacovatinin A.[Pubmed:18310958]
Chem Pharm Bull (Tokyo). 2008 Mar;56(3):398-403.
Total syntheses of (+)-coronarin A (1), (+)-Coronarin E (2), (+)-austrochaparol (3) and (+)-pacovatinin A (4) were achieved from the synthetic (+)-albicanyl acetate (6). Dess-Martin oxidation of (+)-albicanol (5) derived from the chemoenzymatic product (6) gave an aldehyde (7), which was subjected to Julia one-pot olefination using beta-furylmethyl-heteroaromatic sulfones (8 or 9 ) gave (+)-trans Coronarin E (2) and (+)-cis Coronarin E (12) with high cis-selectivity. The synthesis of (+)-coronarin A (1) from (+)-trans Coronarin E (2) was achiev-ed, while (+)-cis Coronarin E (12) was converted to the natural products (+)-(5S,9S,10S)-15,16-epoxy-8(17),13(16),14-labdatriene (13) and (+)-austrochaparol (3). By the asymmetric synthesis of (+)-3, the absolute structure of (+)-3 was determined to be 5S, 7R, 9R, 10S configurations. Homologation of (+)-albicanol (5) followed by allylic oxidation gave (7 alpha)-hydroxy nitrile (17), which was finally converted to the natural (+)-pacovatinin A (4) in 8 steps from (+)-albicanol (5).


