LatifolinCAS# 10154-42-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10154-42-4 | SDF | Download SDF |
| PubChem ID | 340211 | Appearance | Powder |
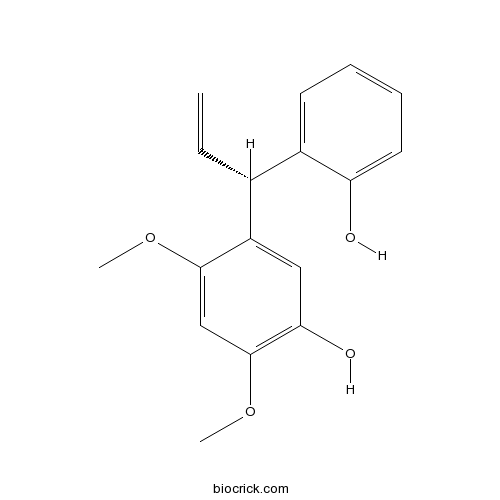
| Formula | C17H18O4 | M.Wt | 286.32 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-[(1R)-1-(2-hydroxyphenyl)prop-2-enyl]-2,4-dimethoxyphenol | ||
| SMILES | COC1=CC(=C(C=C1C(C=C)C2=CC=CC=C2O)O)OC | ||
| Standard InChIKey | OJVQOGDGFIJYPN-LLVKDONJSA-N | ||
| Standard InChI | InChI=1S/C17H18O4/c1-4-11(12-7-5-6-8-14(12)18)13-9-15(19)17(21-3)10-16(13)20-2/h4-11,18-19H,1H2,2-3H3/t11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Latifolin attenuates inflammatory responses by inhibiting NF-κB activation via Nrf2-mediated heme oxygenase-1 expression. 2. Latifolin shows antifungal activity against white- and brown-rot fungi. 3. Latifolin displays potent anticarcinogenic phase II marker enzyme, quinone reductase (QR) inducing activity and high chemopreventive indices. 4. Latifolin is a strong DPPH-scavenger. |
| Targets | HO-1 | NF-kB | COX | NO | PGE | TNF-α | IL Receptor | Nrf2 | IkB | NOS | Antifection | IKK |

Latifolin Dilution Calculator

Latifolin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4926 mL | 17.463 mL | 34.926 mL | 69.8519 mL | 87.3149 mL |
| 5 mM | 0.6985 mL | 3.4926 mL | 6.9852 mL | 13.9704 mL | 17.463 mL |
| 10 mM | 0.3493 mL | 1.7463 mL | 3.4926 mL | 6.9852 mL | 8.7315 mL |
| 50 mM | 0.0699 mL | 0.3493 mL | 0.6985 mL | 1.397 mL | 1.7463 mL |
| 100 mM | 0.0349 mL | 0.1746 mL | 0.3493 mL | 0.6985 mL | 0.8731 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methyl salvionolate A
Catalog No.:BCN3475
CAS No.:1015171-69-3
- 5''-O-Syringoylkelampayoside A
Catalog No.:BCN4798
CAS No.:1014974-98-1
- GSK 0660
Catalog No.:BCC7688
CAS No.:1014691-61-2
- VTP-27999 2,2,2-trifluoroacetate
Catalog No.:BCC2049
CAS No.:1013937-63-7
- CP 376395 hydrochloride
Catalog No.:BCC7604
CAS No.:1013933-37-3
- Gadolinium chloride
Catalog No.:BCC7971
CAS No.:10138-52-0
- Formosanol
Catalog No.:BCN5826
CAS No.:101312-79-2
- PF-04691502
Catalog No.:BCC3837
CAS No.:1013101-36-4
- Zacopride hydrochloride
Catalog No.:BCC7178
CAS No.:101303-98-4
- Noreugenin
Catalog No.:BCN5827
CAS No.:1013-69-0
- PETCM
Catalog No.:BCC2360
CAS No.:10129-56-3
- 11-Chloro-2,3-dihydro-2-methyl-1H- dibenz[2,3:6,7]oxepino[4,5-c]pyrrol-1-one
Catalog No.:BCC8431
CAS No.:1012884-46-6
- Fmoc-D-Pro-OH
Catalog No.:BCC3540
CAS No.:101555-62-8
- Yadanzioside K
Catalog No.:BCN6714
CAS No.:101559-98-2
- Yadanzioside M
Catalog No.:BCN6712
CAS No.:101559-99-3
- trans-2,3-Dihydro-3-ethoxyeuparin
Catalog No.:BCN6923
CAS No.:1015698-14-2
- GSK 4716
Catalog No.:BCC7557
CAS No.:101574-65-6
- 6-O-Nicotinoylbarbatin C
Catalog No.:BCN5828
CAS No.:1015776-92-7
- N-Acetyltryptamine
Catalog No.:BCC6618
CAS No.:1016-47-3
- Trenbolone
Catalog No.:BCC9183
CAS No.:10161-33-8
- Trenbolone acetate
Catalog No.:BCC9184
CAS No.:10161-34-9
- Talipexole
Catalog No.:BCC5250
CAS No.:101626-70-4
- Kadsuracoccinic acid A
Catalog No.:BCN5829
CAS No.:1016260-22-2
- Diacetylpiquerol A
Catalog No.:BCC8935
CAS No.:130466-34-1
Induction of the anticarcinogenic marker enzyme, quinone reductase, by Dalbergiae Lignum.[Pubmed:15473661]
Arch Pharm Res. 2004 Sep;27(9):919-22.
The effect of an extract of Dalbergiae Lignum and four components that were isolated from the extract on the anticarcinogenic phase II marker enzyme, quinone reductase (QR), was investigated. Of the solvent extracts of Dalbergiae Lignum, the CH2Cl2 fraction was the most potent in inducing QR activity, with a CD value (the concentration required to double the QR activity) of 29.5 microg/mL. The CH2Cl2 extract was further separated into six compounds, four of which were identified as 4-methoxydalbergione, Latifolin, 4',6-dihydroxy-7-methoxyflavanone, and obtusafuran. Obtusafuran [CD = 1.1 microM; chemopreventive index (CI) = 101.9] and Latifolin (CD = 1.7 microM; CI = 154.6) displayed potent QR inducing activity and high chemopreventive indices. Latifolin and 4-methoxydalbergione were identified as strong DPPH-scavengers with half-maximal free radical scavenging concentrations of 15.9 and 17.2 microM, respectively.
Bioactivity of latifolin and its derivatives against termites and fungi.[Pubmed:19499920]
J Agric Food Chem. 2009 Jul 8;57(13):5707-12.
Latifolin (1) and its derivatives were investigated with the aim of confirming the correlation between bioactivity (antitermite and antifungal activity) and chemical structure. Termite mortality in response to the derivatives 2'-O-methylLatifolin (2), Latifolin dimethyl ether (4), and Latifolin diacetate (5) increased 2-fold compared to compound 1. The mortality rate from 5-O-methylLatifolin (3) was not different from 1. The mass loss (feed consumption by termite) in response to compounds 3-5 was 3 times greater than compound 1, and the mass loss from compound 2 was twice as great as compound 1. The mortality rate from compounds 4 and 5 increased sharply 7 days after initial exposure. In assessing the antifungal activity of these compounds, it was found that the inhibition rates of white- and brown-rot fungi in response to all derivatives were less than that for compound 1. Our findings indicate that the phenolic hydroxyl group at C-5 of the A ring provides antitermite activities (mortality and mass loss). In addition, both C-5 and C-2' phenolic hydroxyl groups in the A and B rings have antifungal activity against white- and brown-rot fungi. In conclusion, the bioactivity of compound 1 depends upon the position of phenolic hydroxyl groups.
The neoflavonoid latifolin isolated from MeOH extract of Dalbergia odorifera attenuates inflammatory responses by inhibiting NF-kappaB activation via Nrf2-mediated heme oxygenase-1 expression.[Pubmed:24474433]
Phytother Res. 2014 Aug;28(8):1216-23.
In Korea and China, the heartwood of Dalbergia odorifera T. Chen is an important traditional medicine used to treat blood disorders, ischemia, swelling, and epigastric pain. In this study, we investigated the inhibitory effects of Latifolin, a major neoflavonoid component isolated from the MeOH extract of D. odorifera, on the inflammatory reaction of thioglycollate-elicited peritoneal macrophages exposed to lipopolysaccharide, with a particular focus on heme oxygenase-1 (HO-1) expression and nuclear factor-kappaB (NF-kappaB) signaling. Latifolin significantly inhibited the protein and mRNA expression of inducible nitric oxide synthase and COX-2, reduced NO, prostaglandins E2, tumor necrosis factor-alpha, and interleukin-1beta production in primary murine peritoneal macrophages exposed to lipopolysaccharide. Latifolin also suppressed inhibitor kappaB-alpha levels, NF-kappaB nuclear translocation, and NF-kappaB DNA-binding activity. Furthermore, Latifolin upregulated HO-1 expression via nuclear transcription factor-E2-related factor 2 (Nrf2) nuclear translocation. In addition, using inhibitor tin protoporphyrin IX (SnPP), an inhibitor of HO-1, it was verified that the inhibitory effects of Latifolin on the proinflammatory mediators and NF-kappaB DNA-binding activity were associated with the HO-1 expression. These results suggested that the Latifolin-mediated up-regulation of HO-1 expression played a critical role in anti-inflammatory effects in macrophages. This study therefore identified potent therapeutic effects of Latifolin, which warrants further investigation as a potential treatment for inflammatory diseases.


