TrenboloneCAS# 10161-33-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

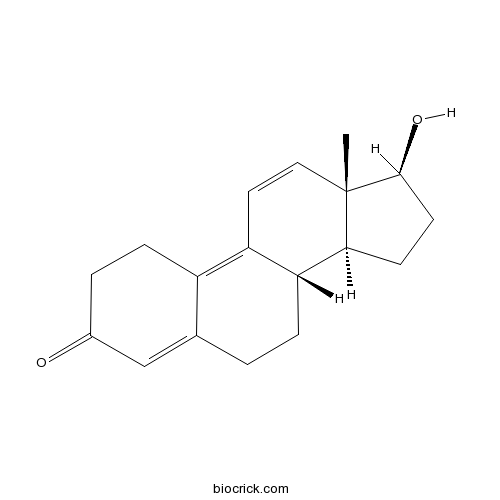
| Cas No. | 10161-33-8 | SDF | Download SDF |
| PubChem ID | 25015 | Appearance | Powder |
| Formula | C18H22O2 | M.Wt | 270.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (8S,13S,14S,17S)-17-hydroxy-13-methyl-2,6,7,8,14,15,16,17-octahydro-1H-cyclopenta[a]phenanthren-3-one | ||
| SMILES | CC12C=CC3=C4CCC(=O)C=C4CCC3C1CCC2O | ||
| Standard InChIKey | MEHHPFQKXOUFFV-OWSLCNJRSA-N | ||
| Standard InChI | InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h8-10,15-17,20H,2-7H2,1H3/t15-,16+,17+,18+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Trenbolone Dilution Calculator

Trenbolone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6982 mL | 18.4911 mL | 36.9822 mL | 73.9645 mL | 92.4556 mL |
| 5 mM | 0.7396 mL | 3.6982 mL | 7.3964 mL | 14.7929 mL | 18.4911 mL |
| 10 mM | 0.3698 mL | 1.8491 mL | 3.6982 mL | 7.3964 mL | 9.2456 mL |
| 50 mM | 0.074 mL | 0.3698 mL | 0.7396 mL | 1.4793 mL | 1.8491 mL |
| 100 mM | 0.037 mL | 0.1849 mL | 0.3698 mL | 0.7396 mL | 0.9246 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-Acetyltryptamine
Catalog No.:BCC6618
CAS No.:1016-47-3
- 6-O-Nicotinoylbarbatin C
Catalog No.:BCN5828
CAS No.:1015776-92-7
- GSK 4716
Catalog No.:BCC7557
CAS No.:101574-65-6
- trans-2,3-Dihydro-3-ethoxyeuparin
Catalog No.:BCN6923
CAS No.:1015698-14-2
- Yadanzioside M
Catalog No.:BCN6712
CAS No.:101559-99-3
- Yadanzioside K
Catalog No.:BCN6714
CAS No.:101559-98-2
- Fmoc-D-Pro-OH
Catalog No.:BCC3540
CAS No.:101555-62-8
- Latifolin
Catalog No.:BCN7778
CAS No.:10154-42-4
- Methyl salvionolate A
Catalog No.:BCN3475
CAS No.:1015171-69-3
- 5''-O-Syringoylkelampayoside A
Catalog No.:BCN4798
CAS No.:1014974-98-1
- GSK 0660
Catalog No.:BCC7688
CAS No.:1014691-61-2
- VTP-27999 2,2,2-trifluoroacetate
Catalog No.:BCC2049
CAS No.:1013937-63-7
- Trenbolone acetate
Catalog No.:BCC9184
CAS No.:10161-34-9
- Talipexole
Catalog No.:BCC5250
CAS No.:101626-70-4
- Kadsuracoccinic acid A
Catalog No.:BCN5829
CAS No.:1016260-22-2
- Diacetylpiquerol A
Catalog No.:BCC8935
CAS No.:130466-34-1
- Sodium Monofluorophosphate
Catalog No.:BCC4722
CAS No.:10163-15-2
- Drevogenin A
Catalog No.:BCN4740
CAS No.:10163-83-4
- Cyclo(Hpro-Leu)
Catalog No.:BCN2430
CAS No.:1016899-93-6
- Cyclo(Phe-Hpro)
Catalog No.:BCN2425
CAS No.:1016899-94-7
- Barpisoflavone A
Catalog No.:BCN4739
CAS No.:101691-27-4
- Olivil monoacetate
Catalog No.:BCN4738
CAS No.:1016974-78-9
- Sulfocostunolide A
Catalog No.:BCN5830
CAS No.:1016983-51-9
- 6-O-p-Hydroxybenzoylaucubin
Catalog No.:BCN5831
CAS No.:1016987-87-3
TMT-Based Proteomics Profiling of Bovine Liver Underscores Protein Markers of Anabolic Treatments.[Pubmed:30865377]
Proteomics. 2019 Mar 13:e1800422.
Illegal use of pharmacologically active compounds for growth promotion in food production exposes consumers to health risk. Surveillance of such practices is based on direct detection of drugs or related metabolites by LC-MS/MS. Screening strategies focusing on indirect biological responses are considered promising tools to improve surveillance. In the present study, an untargeted shot-gun proteomics approach based on tandem mass tags (TMT) was carried out to identify proteins altered in bovine liver after different anabolic treatments. Three controlled pharmacological treatments with dexamethasone alone, dexamethasone and clenbuterol, or a combination of Trenbolone and estradiol were analyzed. Untargeted TMT analysis of liver digests by LC-HRMS/MS allowed for the simultaneous identification and relative quantification of proteins. Thanks to PLS-DA we identified a set of proteins capable to classify animals treated with dexamethasone alone (11 proteins), or in combination with clenbuterol (13 proteins). No significant difference was found upon administration of sexual steroids. Protein markers were relatively quantified using targeted proteomics by PRM. Two predictive models were trained to validate protein markers. Finally, an independent animal set of 12 bulls (4 controls and 8 treated with dexamethasone) was analyzed by PRM to further validate one of the predictive models giving an accuracy of 100%. This article is protected by copyright. All rights reserved.
Context-specific behavioural changes induced by exposure to an androgenic endocrine disruptor.[Pubmed:30743111]
Sci Total Environ. 2019 May 10;664:177-187.
Pharmaceutical contaminants are being detected with increased frequency in organisms and ecosystems worldwide. This represents a major environmental concern given that various pharmaceuticals act on drug targets that are evolutionarily conserved across diverse taxa, are often persistent in the environment, and can bioconcentrate in organisms and bioaccumulate in food chains. Despite this, relatively little is known about the potential for pharmaceutical contaminants to affect animal behaviour, especially across multiple fitness-related contexts. Here, we investigated impacts of 21-day exposure of wild-caught male eastern mosquitofish (Gambusia holbrooki) to a field-realistic level of the veterinary pharmaceutical 17beta-Trenbolone-a growth-promoting steroid used extensively in beef production worldwide and a potent androgenic endocrine disruptor repeatedly detected in surface waters affected by livestock effluent run-off. First, we examined male boldness, activity, and exploratory behaviour in a novel environment (maze arena) and found no significant effect of 17beta-Trenbolone exposure. Second, the same males were tested in a reproductive assay for their tendency to associate with a stimulus (unexposed) female behind a partition. Exposed males exhibited reduced association behaviour, taking longer to first associate with, and spending less time within close proximity to, a female. Third, all males were assayed for sperm function (computer-assisted sperm analysis, sperm viability) or quantity (total sperm count) and, although no significant main effects of 17beta-Trenbolone were seen on sperm traits, exposure altered the relationship between male morphology and sperm function. Lastly, morphological traits were assessed and exposed males were found to have, on average, increased mass relative to length. In combination, these results demonstrate that exposure to a field-realistic level of 17beta-Trenbolone can produce subtle but important trait alterations in male fish-including context-specific behavioural changes, disruption of key sperm function trade-offs, and altered morphology-with potential impacts on exposed wildlife.
Assessment of human estrogen receptor agonistic/antagonistic effects of veterinary drugs used for livestock and farmed fish by OECD in vitro stably transfected transcriptional activation assays.[Pubmed:30742918]
Toxicol In Vitro. 2019 Feb 10. pii: S0887-2333(18)30384-9.
The presence of veterinary drug residues in foods and the environment could potentially cause adverse effects on humans and wildlife. Several veterinary drugs were reported to exhibit endocrine disrupting effects via binding affinities to sexual hormone receptors such as estrogen and androgen receptors. Therefore, we confirmed the human estrogen receptor (ER) agonistic/antagonistic effects of 135 chemicals that were used as veterinary drugs in Korea by the official Organization for Economic Cooperation and Development (OECD) in vitro ER transcriptional activation (TA) assay using the VM7Luc4E2 cell line. In the case of ER agonist screening, 7 veterinary drugs (cefuroxime, cymiazole, Trenbolone, zeranol, phoxim, altrenogest and nandrolone) were determined to be ER agonists. In addition, only zeranol was found to exhibit weak ER antagonistic activity. These 7 veterinary drugs, which were determined as ER agonists and/or antagonists by an OECD in vitro assay, were also found to have binding affinity to ERs. These results indicate that various veterinary drugs possess potential (anti-)estrogenic effects. However, further study is needed to determine the precise endocrine-disrupting effects of these compounds.
Gonadosomatic index as a confounding variable in fish-based screening assays for the detection of anti-estrogens and nonaromatizable androgens.[Pubmed:30614037]
Environ Toxicol Chem. 2019 Mar;38(3):603-615.
The presence of reproductive endocrine-disrupting compounds (REDCs) in the environment poses a potential threat to fish and wildlife, because exposures are capable of altering sexual development, reproductive success, and behavior. Fish-based screening assays are often utilized to screen for the presence of REDCs in surface waters and to assess single chemicals for potential endocrine-disrupting activity. In an effort to improve such screening assays, the goal of the present study was to determine whether the gonadosomatic index (GSI) of female fathead minnows (Pimephales promelas), as assessed via external characteristics, influences their response to REDC exposure. Specifically, we sought to determine whether low-GSI females differed from high-GSI females in their responses to the model anti-estrogen fadrozole and the model androgen 17beta-Trenbolone, and whether there was a preferable classification in the context of REDC screening. Low-GSI females were more sensitive to fadrozole at the lower concentration of fadrozole (5 microg/L) and to the higher concentration of Trenbolone (50 ng/L), whereas high-GSI females were more sensitive at the lower concentration of Trenbolone (5 ng/L). The differential response of low- and high-GSI females to REDCs indicates that GSI influences exposure outcome, and should subsequently be taken into consideration in the implementation of screening assays, as failure to utilize fish of the appropriate reproductive status may skew the test results. Environ Toxicol Chem 2019;38:603-615. (c) 2019 SETAC.
In vitro-to-in vivo extrapolation (IVIVE) by PBTK modeling for animal-free risk assessment approaches of potential endocrine-disrupting compounds.[Pubmed:30552464]
Arch Toxicol. 2019 Feb;93(2):401-416.
While in vitro testing is used to identify hazards of chemicals, nominal in vitro assay concentrations may misrepresent potential in vivo effects and do not provide dose-response data which can be used for a risk assessment. We used reverse dosimetry to compare in vitro effect concentrations-to-in vivo doses causing toxic effects related to endocrine disruption. Ten compounds (acetaminophen, bisphenol A, caffeine, 17alpha-ethinylestradiol, fenarimol, flutamide, genistein, ketoconazole, methyltestosterone, and Trenbolone) have been tested in the yeast estrogen screening (YES) or yeast androgen-screening (YAS) assays for estrogen and androgen receptor binding, as well as the H295R assay (OECD test guideline no. 456) for potential interaction with steroidogenesis. With the assumption of comparable concentration-response ratios of these effects in the applied in vitro systems and the in vivo environment, the lowest observed effect concentrations from these assays were extrapolated to oral doses (LOELs) by reverse dosimetry. For extrapolation, an eight-compartment Physiologically Based Toxicokinetic (PBTK) rat model based on in vitro and in silico input data was used. The predicted LOEL was then compared to the LOEL actually observed in corresponding in vivo studies (YES/YAS assay versus uterotrophic or Hershberger assay and steroidogenesis assay versus pubertal assay or generation studies). This evaluation resulted in 6 out of 10 compounds for which the predicted LOELs were in the same order of magnitude as the actual in vivo LOELs. For four compounds, the predicted LOELs differed by more than tenfold from the actual in vivo LOELs. In conclusion, these data demonstrate the applicability of reverse dosimetry using a simple PBTK model to serve in vitro-in silico-based risk assessment, but also identified cases and test substance were the applied methods are insufficient.
Toxic Impact of Anabolic Androgenic Steroids in Primary Rat Cortical Cell Cultures.[Pubmed:30500611]
Neuroscience. 2019 Jan 15;397:172-183.
The use of anabolic androgenic steroids (AASs) among non-athletes is a public health-problem, as abusers underestimate the negative effects associated with these drugs. The present study investigated the toxic effects of testosterone, nandrolone, stanozolol, and Trenbolone, and aimed to understand how AAS abuse affects the brain. Mixed cortical cultures from embryonic rats were grown in vitro for 7days and thereafter treated with increasing concentrations of AASs for 24h (single-dose) or 3days (repeated exposure). Cells were co-treated with the androgen-receptor (AR) antagonist flutamide, to determine whether the potential adverse effects observed were mediated by the AR. Cellular toxicity was determined by measuring mitochondrial activity, lactate dehydrogenase (LDH) release, and caspase-3/7 activity. Nandrolone, unlike the other AASs studied, indicated an effect on mitochondrial activity after 24h. Furthermore, single-dose exposure with testosterone, nandrolone and Trenbolone increased LDH release, while no effect was detected with stanozolol. However, all of the four steroids negatively affected mitochondrial function and resulted in LDH release after repeated exposure. Testosterone, nandrolone, and Trenbolone caused their toxic effects by induction of apoptosis, unlike stanozolol that seemed to induce necrosis. Flutamide almost completely prevented AAS-induced toxicity by maintaining mitochondrial function, cellular integrity, and inhibition of apoptosis. Overall, we found that supra-physiological concentrations of AASs induce cell death in mixed primary cortical cultures, but to different extents, and possibly through various mechanisms. The data presented herein suggest that the molecular interactions of the AASs with the AR are primarily responsible for the toxic outcomes observed.
Fish on steroids: Temperature-dependent effects of 17beta-trenbolone on predator escape, boldness, and exploratory behaviors.[Pubmed:30423539]
Environ Pollut. 2019 Feb;245:243-252.
Hormonal growth promoters (HGPs), widely used in beef cattle production globally, make their way into the environment as agricultural effluent-with potential impacts on aquatic ecosystems. One HPG of particular concern is 17beta-Trenbolone, which is persistent in freshwater habitats and can affect the development, morphology and reproductive behaviors of aquatic organisms. Despite this, few studies have investigated impacts of 17beta-Trenbolone on non-reproductive behaviors linked to growth and survival, like boldness and predator avoidance. None consider the interaction between 17beta-Trenbolone and other environmental stressors, such as temperature, although environmental challenges confronting animals in the wild seldom, if ever, occur in isolation. Accordingly, this study aimed to test the interactive effects of Trenbolone and temperature on organismal behavior. To do this, eastern mosquitofish (Gambusia holbrooki) were subjected to an environmentally-relevant concentration of 17beta-Trenbolone (average measured concentration 3.0+/-0.2ng/L) or freshwater (i.e. control) for 21 days under one of two temperatures (20 and 30 degrees C), after which the predator escape, boldness and exploration behavior of fish were tested. Predator escape behavior was assayed by subjecting fish to a simulated predator strike, while boldness and exploration were assessed in a separate maze experiment. We found that Trenbolone exposure increased boldness behavior. Interestingly, some behavioral effects of Trenbolone depended on temperature, sex, or both. Specifically, significant effects of Trenbolone on male predator escape behavior were only noted at 30 degrees C, with males becoming less reactive to the simulated threat. Further, in the maze experiment, Trenbolone-exposed fish explored the maze faster than control fish, but only at 20 degrees C. We conclude that field detected concentrations of 17beta-Trenbolone can impact ecologically important behaviors of fish, and such effects can be temperature dependent. Such findings underscore the importance of considering the potentially interactive effects of other environmental stressors when investigating behavioral effects of environmental contaminants.
Field-realistic exposure to the androgenic endocrine disruptor 17beta-trenbolone alters ecologically important behaviours in female fish across multiple contexts.[Pubmed:30245452]
Environ Pollut. 2018 Dec;243(Pt B):900-911.
The capacity of pharmaceutical pollution to alter behaviour in wildlife is of increasing environmental concern. A major pathway of these pollutants into the environment is the treatment of livestock with hormonal growth promotants (HGPs), which are highly potent veterinary pharmaceuticals that enter aquatic ecosystems via effluent runoff. Hormonal growth promotants are designed to exert biological effects at low doses, can act on physiological pathways that are evolutionarily conserved across taxa, and have been detected in ecosystems worldwide. However, despite being shown to alter key fitness-related processes (e.g., development, reproduction) in various non-target species, relatively little is known about the potential for HGPs to alter ecologically important behaviours, especially across multiple contexts. Here, we investigated the effects of exposure to a field-realistic level of the androgenic HGP metabolite 17beta-Trenbolone-an endocrine-disrupting chemical that has repeatedly been detected in freshwater systems-on a suite of ecologically important behaviours in wild-caught female eastern mosquitofish (Gambusia holbrooki). First, we found that 17beta-Trenbolone-exposed fish were more active and exploratory in a novel environment (i.e., maze arena), while boldness (i.e., refuge use) was not significantly affected. Second, when tested for sociability, exposed fish spent less time in close proximity to a shoal of stimulus (i.e., unexposed) conspecific females and were, again, found to be more active. Third, when assayed for foraging behaviour, exposed fish were faster to reach a foraging zone containing prey items (chironomid larvae), quicker to commence feeding, spent more time foraging, and consumed a greater number of prey items, although the effect of exposure on certain foraging behaviours was dependent on fish size. Taken together, these findings highlight the potential for exposure to sub-lethal levels of veterinary pharmaceuticals to alter sensitive behavioural processes in wildlife across multiple contexts, with potential ecological and evolutionary implications for exposed populations.
Oxytocin is involved in steroid hormone-stimulated bovine satellite cell proliferation and differentiation in vitro.[Pubmed:30195176]
Domest Anim Endocrinol. 2019 Jan;66:1-13.
Sex steroid hormones are used in the meat industry due to their ability to regulate muscle hypertrophy. However, the mechanisms underlying their action are not fully elucidated. Recent reports demonstrate that steroid hormones increase oxytocin (OXT) expression in skeletal muscle, indicating that OXT may play a role in satellite cell activity. This hypothesis was tested using steroid hormones (17beta-estradiol [E2]; Trenbolone acetate [TBA]), tamoxifen (TAM), OXT, and atosiban (A: OXT receptor inhibitor) applied to bovine satellite cells (BSCs) to investigate BSC regulation by OXT. Oxytocin alone increased fusion index (P < 0.05) but not BSC proliferation. Oxytocin reduced (P < 0.05) apoptotic nuclei and stimulated migration rate (P < 0.05). Similarly, E2 and TBA increased (P < 0.05) BSC proliferation rate, fusion index, and migration and decreased (P < 0.05) apoptotic nuclei. 17beta-Estradiol or TBA supplemented with A had lower (P < 0.05) BSC proliferation rate, fusion index, and migration and more (P < 0.05) apoptotic nuclei compared with E2 or TBA alone. Furthermore, OXT expression increased (P < 0.05) in E2 or TBA-treated proliferating BSC. Oxytocin, E2, and TBA increased (P < 0.05) MyoD and MyoG expression in proliferating BSC. During BSC differentiation, OXT expression increased (P < 0.05) with E2 or TBA treatments. MyoG expression increased (P < 0.05) in OXT, E2, and TBA compared with control. However, A, OXT + A, TAM, TAM + OXT, E2 + TAM, E2 + A, and TBA + A decreased (P < 0.05) MyoG expression during BSC differentiation. These results indicate that OXT is involved in steroid hormone-stimulated BSC activity.
Acute pancreatitis secondary to the use of the anabolic steroid trenbolone acetate.[Pubmed:30101635]
Clin Toxicol (Phila). 2019 Jan;57(1):60-62.
BACKGROUND: The use of performance-enhancing drugs has increased dramatically in the last decade with high prevalence reported among the young athlete population. Many of these drugs contain anabolic steroids and may carry potential significant side effects and health risks. We report a case of anabolic steroid-induced acute pancreatitis (AP) that recurred after the reuse of the same drug by the patient, confirming the causative relationship. CASE REPORT: A 24 year-old male presented with severe epigastric pain. His past medical history was significant for two hospitalizations during the last year with AP. During his hospital admissions, extensive workup was performed ruling out the common and uncommon causes of AP. Upon further pressing, the patient admitted to a history of past and current anabolic steroid use for athletic performance enhancement. He began this use four years ago and most recently started using Trenbolone acetate (TA). The correlation between the timing of the anabolic steroids administration and the attacks of AP, along with ruling out other causes, confirmed TA as the cause of pancreatitis. DISCUSSION: The side effects associated with the use of these increasingly prevalent drugs are difficult to study in clinical trials due to the unethical nature of their consumption. In addition, these medications are difficult to study due to the varied usage cycles and patterns, unknown origin and source, as well as often high dose ingestion. Physicians and body builders need to be aware of the possible serious consequences of their use.
Vitamin A administration at birth promotes calf growth and intramuscular fat development in Angus beef cattle.[Pubmed:30062009]
J Anim Sci Biotechnol. 2018 Jul 23;9:55.
Background: Marbling, or intramuscular fat, is an important factor contributing to the palatability of beef. Vitamin A, through its active metabolite, retinoic acid, promotes the formation of new fat cells (adipogenesis). As intramuscular adipogenesis is active during the neonatal stage, we hypothesized that vitamin A administration during the neonatal stage would enhance intramuscular adipogenesis and marbling. Methods: Angus steer calves (n = 30), in a completely randomized design, were randomly allotted to three treatment groups at birth, receiving 0, 150,000, or 300,000 IU of vitamin A at both birth and one month of age. A biopsy of the biceps femoris muscle was collected at two months of age. After weaning at 210 d of age, steers were fed a backgrounding diet in a feedlot until 308 d of age, when they were transitioned to a high concentrate finishing diet and implanted with Trenbolone/estradiol/tylosin mixture. Steers were harvested at an average of 438 d of age. All diets were formulated to meet nutrient requirements. Results: Weaning weight and weight during the backgrounding phase were linearly increased (P < 0.05) by vitamin A level, though no difference in body weight was observed at harvest. Intramuscular fat of steers at 308 d of age, measured by ultrasound, quadratically increased (P < 0.05) with vitamin A level from 4.0+/-0.26 % to 4.9+/-0.26 %. Similarly, carcass marbling score in the ribeye quadratically increased (P < 0.05). Conclusion: Administration of vitamin A at birth increased weaning weight and enhanced marbling fat development. Thus, vitamin A administration provides a practical method for increasing marbling and early growth of beef cattle.
(1)H NMR determination of adulteration of anabolic steroids in seized drugs.[Pubmed:30003910]
Steroids. 2018 Oct;138:47-56.
Counterfeiting and adulteration of pharmaceuticals is a prevalent problem worldwide and represents a major health risk to the population, with anabolic steroids being one of the main classes of drugs consumed and obtained from dubious sources. In this work, we propose the use of the (1)H NMR technique to evaluate formulations containing anabolic steroids, with analysis of 40 samples of anabolic drugs that are used in injectable and capsule forms. The samples analyzed presented the following active ingredients: testosterone propionate, testosterone phenylpropionate, testosterone isocaproate, testosterone decanoate, testosterone cypionate, testosterone undecanoate, stanozolol, drostanolone propionate, Trenbolone acetate, oxymetholone, and methandrostenolone. The (1)H NMR spectroscopic measurements were performed using a 600MHz Bruker Avance III spectrometer, with deuterated chloroform (CDCl3) containing 0.1% TMS as solvent. Of the 40 samples analyzed, eight did not show the presence of the active principle stated on the label. Three types of adulteration were found in the analyzed samples: absence of the active ingredient, adulteration with other substances, and concentration values below those indicated on the label. Sildenafil citrate was found in four samples. The GC-MS technique was used to confirm the adulteration results found using (1)H NMR. Quantitative determination by NMR was performed using internal standard and ERETIC 2 methods, and the results obtained were statistically the same.


