NoreugeninCAS# 1013-69-0 |
Quality Control & MSDS
Number of papers citing our products

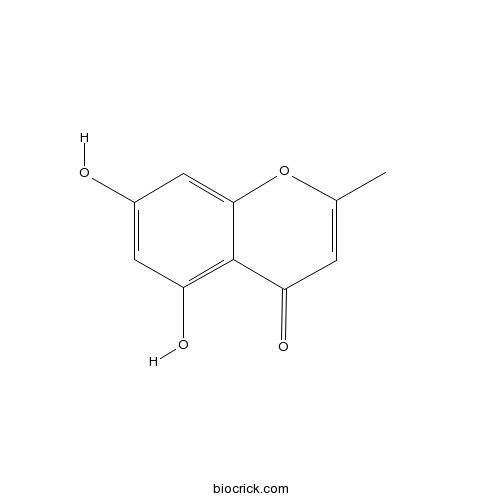
Chemical structure

3D structure
| Cas No. | 1013-69-0 | SDF | Download SDF |
| PubChem ID | 5375252 | Appearance | Powder |
| Formula | C10H8O4 | M.Wt | 192.2 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 250 mg/mL (1300.93 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 5,7-dihydroxy-2-methylchromen-4-one | ||
| SMILES | CC1=CC(=O)C2=C(C=C(C=C2O1)O)O | ||
| Standard InChIKey | NCUJRUDLFCGVOE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H8O4/c1-5-2-7(12)10-8(13)3-6(11)4-9(10)14-5/h2-4,11,13H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Chromone glycosides from Knoxia corymbosa.[Pubmed: 17135054]J Asian Nat Prod Res. 2006 Oct-Nov;8(7):663-70.Four new chromone glycosides, corymbosins K1-K4 (3-6), together with two known compounds, Noreugenin (1) and undulatoside A (2), were isolated from the whole plant of Knoxiacorymbosa (Rubiaceae). |
| Structure Identification | Planta Med. 1988 Jun;54(3):239-42.New Chromone Alkaloids from the Stem Bark of Schumanniophyton magnificum.[Pubmed: 17265261]Fractionation of a methanolic extract of the stem bark of SCHUMANNIOPHYTON MAGNIFICUM yielded large quantities of mannitol. In addition, Noreugenin and ten related chromone alkaloids were isolated. Seven of these alkaloids had been isolated previously from S. MAGNIFICUM and one other from S.PROBLEMATICUM but two of the alkaloids were novel, one was hydroxy- N-methylschumannificine and the other was the acetate of N-demethylrohitukine. The structures of the two alkaloids have been deduced from their spectral features. |

Noreugenin Dilution Calculator

Noreugenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2029 mL | 26.0146 mL | 52.0291 mL | 104.0583 mL | 130.0728 mL |
| 5 mM | 1.0406 mL | 5.2029 mL | 10.4058 mL | 20.8117 mL | 26.0146 mL |
| 10 mM | 0.5203 mL | 2.6015 mL | 5.2029 mL | 10.4058 mL | 13.0073 mL |
| 50 mM | 0.1041 mL | 0.5203 mL | 1.0406 mL | 2.0812 mL | 2.6015 mL |
| 100 mM | 0.052 mL | 0.2601 mL | 0.5203 mL | 1.0406 mL | 1.3007 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PETCM
Catalog No.:BCC2360
CAS No.:10129-56-3
- 11-Chloro-2,3-dihydro-2-methyl-1H- dibenz[2,3:6,7]oxepino[4,5-c]pyrrol-1-one
Catalog No.:BCC8431
CAS No.:1012884-46-6
- Phenserine
Catalog No.:BCC7529
CAS No.:101246-66-6
- Kushenol M
Catalog No.:BCN3310
CAS No.:101236-51-5
- Kushenol L
Catalog No.:BCN3309
CAS No.:101236-50-4
- Kushenol K
Catalog No.:BCN3448
CAS No.:101236-49-1
- IRAK inhibitor 3
Catalog No.:BCC1656
CAS No.:1012343-93-9
- Picrasidine Q
Catalog No.:BCN3182
CAS No.:101219-61-8
- IRAK inhibitor 4
Catalog No.:BCC1657
CAS No.:1012104-68-5
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- Longipedlactone J
Catalog No.:BCN6644
CAS No.:1011762-93-8
- Momordicoside P
Catalog No.:BCN3275
CAS No.:1011726-62-7
- Zacopride hydrochloride
Catalog No.:BCC7178
CAS No.:101303-98-4
- PF-04691502
Catalog No.:BCC3837
CAS No.:1013101-36-4
- Formosanol
Catalog No.:BCN5826
CAS No.:101312-79-2
- Gadolinium chloride
Catalog No.:BCC7971
CAS No.:10138-52-0
- CP 376395 hydrochloride
Catalog No.:BCC7604
CAS No.:1013933-37-3
- VTP-27999 2,2,2-trifluoroacetate
Catalog No.:BCC2049
CAS No.:1013937-63-7
- GSK 0660
Catalog No.:BCC7688
CAS No.:1014691-61-2
- 5''-O-Syringoylkelampayoside A
Catalog No.:BCN4798
CAS No.:1014974-98-1
- Methyl salvionolate A
Catalog No.:BCN3475
CAS No.:1015171-69-3
- Latifolin
Catalog No.:BCN7778
CAS No.:10154-42-4
- Fmoc-D-Pro-OH
Catalog No.:BCC3540
CAS No.:101555-62-8
- Yadanzioside K
Catalog No.:BCN6714
CAS No.:101559-98-2
Chromone glycosides from Knoxia corymbosa.[Pubmed:17135054]
J Asian Nat Prod Res. 2006 Oct-Nov;8(7):663-70.
Four new chromone glycosides, corymbosins K1-K4 (3-6), together with two known compounds, Noreugenin (1) and undulatoside A (2), were isolated from the whole plant of Knoxiacorymbosa (Rubiaceae). The structures of the new compounds were established through extensive NMR or X-ray spectroscopic analysis as 7-O-beta-D-allopyranosyl-5-hydroxy-2-methylchromone (corymbosin K1, 3), 7-O-beta-D-6-acetylglucopyranosyl-5-hydroxy-2-methylchromone (corymbosin K2, 4), 7-O-[6-O-(4-O-trans-caffeoyl-beta-D-allopyranosyl)]-beta-D-glucopyranosyl-5-hydro xy-2-methylchromone (corymbosin K3, 5) and 7-O-[6-O-(4-O-trans-feruloyl-beta-D-allopyranosyl)]-beta-D-glucopyranosyl-5-hydro xy-2- methylchromone (corymbosin K4, 6). Compounds 2-5 were subjected to test their immunomodulatory activity invitro.
New Chromone Alkaloids from the Stem Bark of Schumanniophyton magnificum.[Pubmed:17265261]
Planta Med. 1988 Jun;54(3):239-42.
Fractionation of a methanolic extract of the stem bark of SCHUMANNIOPHYTON MAGNIFICUM yielded large quantities of mannitol. In addition, Noreugenin and ten related chromone alkaloids were isolated. Seven of these alkaloids had been isolated previously from S. MAGNIFICUM and one other from S.PROBLEMATICUM but two of the alkaloids were novel, one was hydroxy- N-methylschumannificine and the other was the acetate of N-demethylrohitukine. The structures of the two alkaloids have been deduced from their spectral features.


