LY 2158405-HT2/5-HT7 antagonist CAS# 137328-52-0 |
- T0901317
Catalog No.:BCC1178
CAS No.:293754-55-9
- GW3965
Catalog No.:BCC1612
CAS No.:405911-09-3
- GW3965 HCl
Catalog No.:BCC3790
CAS No.:405911-17-3
- Fexaramine
Catalog No.:BCC7412
CAS No.:574013-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 137328-52-0 | SDF | Download SDF |
| PubChem ID | 132049 | Appearance | Powder |
| Formula | C24H33N3O2 | M.Wt | 395.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 25 mM in ethanol | ||
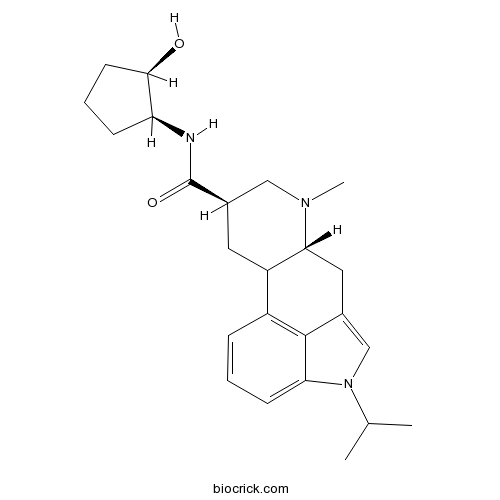
| Chemical Name | (6aR,9R)-N-[(1S,2R)-2-hydroxycyclopentyl]-7-methyl-4-propan-2-yl-6,6a,8,9,10,10a-hexahydroindolo[4,3-fg]quinoline-9-carboxamide | ||
| SMILES | CC(C)N1C=C2CC3C(CC(CN3C)C(=O)NC4CCCC4O)C5=C2C1=CC=C5 | ||
| Standard InChIKey | IMSDOBUYDTVEHN-LUKLFJNJSA-N | ||
| Standard InChI | InChI=1S/C24H33N3O2/c1-14(2)27-13-15-11-21-18(17-6-4-8-20(27)23(15)17)10-16(12-26(21)3)24(29)25-19-7-5-9-22(19)28/h4,6,8,13-14,16,18-19,21-22,28H,5,7,9-12H2,1-3H3,(H,25,29)/t16-,18?,19+,21-,22-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-HT2/5-HT7 receptor antagonist. Reduces stimulatory effect of serotonin on aldosterone secretion; also reduces 5-HT-induced cAMP formation (pKB = 8.26). |

LY 215840 Dilution Calculator

LY 215840 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5282 mL | 12.6409 mL | 25.2819 mL | 50.5638 mL | 63.2047 mL |
| 5 mM | 0.5056 mL | 2.5282 mL | 5.0564 mL | 10.1128 mL | 12.6409 mL |
| 10 mM | 0.2528 mL | 1.2641 mL | 2.5282 mL | 5.0564 mL | 6.3205 mL |
| 50 mM | 0.0506 mL | 0.2528 mL | 0.5056 mL | 1.0113 mL | 1.2641 mL |
| 100 mM | 0.0253 mL | 0.1264 mL | 0.2528 mL | 0.5056 mL | 0.632 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cathepsin S inhibitor
Catalog No.:BCC1455
CAS No.:1373215-15-6
- Dehydroeffusol
Catalog No.:BCN2927
CAS No.:137319-34-7
- 4,5-Dioxo-4,5-seco-11(13)-cadinen-12-oic acid
Catalog No.:BCN1577
CAS No.:137288-61-0
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid
Catalog No.:BCC8670
CAS No.:137281-39-1
- Pemetrexed
Catalog No.:BCC9115
CAS No.:137281-23-3
- Boc-Gln-OH
Catalog No.:BCC3382
CAS No.:13726-85-7
- Boc-Glu(OtBu)-OH
Catalog No.:BCC3392
CAS No.:13726-84-6
- Boc-Hyp-OH
Catalog No.:BCC3251
CAS No.:13726-69-7
- Boc-Asp-OH
Catalog No.:BCC2609
CAS No.:13726-67-5
- Chlorantholide E
Catalog No.:BCN4836
CAS No.:1372558-36-5
- Chlorantholide C
Catalog No.:BCN4837
CAS No.:1372558-35-4
- Chlorantholide B
Catalog No.:BCN4834
CAS No.:1372558-34-3
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- Boc-Lys-OH
Catalog No.:BCC3410
CAS No.:13734-28-6
- Boc-Phe-OH
Catalog No.:BCC3432
CAS No.:13734-34-4
- Boc-Sar-OH
Catalog No.:BCC3337
CAS No.:13734-36-6
- Boc-Ser(tBu)-OH
Catalog No.:BCC3444
CAS No.:13734-38-8
- Boc-Thr(tBu)-OH
Catalog No.:BCC3452
CAS No.:13734-40-2
- Boc-Val-OH
Catalog No.:BCC3465
CAS No.:13734-41-3
- GSK J1
Catalog No.:BCC2231
CAS No.:1373422-53-7
- GSK J4 HCl
Catalog No.:BCC2230
CAS No.:1373423-53-0
- Nω-Propyl-L-arginine hydrochloride
Catalog No.:BCC6965
CAS No.:137361-05-8
- PF-5274857
Catalog No.:BCC3838
CAS No.:1373615-35-0
- Spathulatol
Catalog No.:BCN6877
CAS No.:1373888-27-7
Clinical and immunohistochemical performance of lyophilized platelet-rich fibrin (Ly-PRF) on tissue regeneration.[Pubmed:28192870]
Clin Implant Dent Relat Res. 2017 Jun;19(3):466-477.
BACKGROUND: Platelet-rich fibrin (PRF) has been widely used in oral implantology and other fields, but benefits of the fresh PRF (FPRF (fresh platelet-rich fibrin)) were consequently limited because of its short-term application. Thus, a protocol for the combination of PRF and lyophilization comes up in the present study to address the issue of PRF storage and delayed clinical application, which has little been reported in this field at home and abroad by now. PURPOSE: The aim of the present study was to evaluate the applicability of lyophilized platelet-rich fibrin (Ly-PRF) used as the scaffold material for craniofacial tissue regeneration and to compare its biochemical properties with commonly used fresh PRF. MATERIALS AND METHODS: Two volunteers with both genders were selected as the source of PRF and Ly-PRF samples. Macro- and micro-scopic appearance evaluation as well as immunohistochemical comparison were performed on PRF samples before and after freeze-drying at -196 degrees C. The second experimental phase was to observe clinical performance when fresh and lyophilized PRF were applied in guided bone regeneration (GBR) operations in 39 patients losing teeth in the anterior maxillary region who required an oral implantation followed by labial bone grafting. RESULTS: The conventional histological and transmission electron microscopy images showed the microstructure of Ly-PRF, which resembled a mesh containing apparently irregularly shaped platelets with less alpha-granule than fresh PRF in micro and a translucent membrane with less elasticity than fresh PRF in macro. Simultaneous immunohistological staining results showed positive expression of PDGF-BB, IL-1, IL-4, TNF, TGF-beta1 in both fresh and lyophilized PRF, while the expression of PDGF-BB, IL-1, TNF, TGF-beta1 has no statistical difference between them (P > .05) but that of IL-4 in Ly-PRF is statistically higher than in fresh PRF (P < .05). When applied in GBR operations, there were no significant differences between Ly-PRF and FPRF in factors of histological and clinical evaluations (i.e., color, swelling, bleeding of the mucosa, pain leveland, and remodeling of hard tissue) performed 3 days, 7 days, and 4 months after the surgery (P > .05). CONCLUSIONS: This study strongly supports that lyophilization at -196 degrees C does not largely influence the expression of bioactive factors, the microstructure of fibrinogen or the clinical effects of PRF.
Expansion of CD11b(+)Ly-6C(+) myeloid-derived suppressor cells (MDSCs) driven by galectin-9 attenuates CVB3-induced myocarditis.[Pubmed:28110209]
Mol Immunol. 2017 Mar;83:62-71.
Galectin-9 is known to play a role in the modulation of innate and adaptive immunity to ameliorate CVB3-induced myocarditis. In the present study, we found that galectin-9 induced the expansion of CD11b(+)Ly-6C(+) myeloid-derived suppressor cells (MDSCs) in the heart from CVB3-infected mice. Adoptive transfer of CD11b(+)Ly-6C(+) MDSCs significantly alleviated myocarditis accompanied by increased Th2 and Treg frequency and anti-inflammatory cytokines expression in the heart tissue. Moreover, Ly6C(+) MDSCs, but not Ly6G(+) cells, expressed Arg-1 and NOS2, and suppressed CD4(+) T cell proliferation in vitro in an Arg-1-dependent mechanism; an event that was reversed with treatment of either an Arg-1 inhibitor or addition of excess l-arginine. Furthermore, Ly6C(+) MDSCs co-expressed higher levels of F4/80, Tim-3, and IL-4Ralpha, and had the plasticity to up-regulate NOS2 or Arg-1 in response to IFN-gamma or IL-4 treatment. The present results indicate that galectin-9 expands CD11b(+)Ly-6C(+) MDSCs to ameliorate CVB3-induced myocarditis.
Assessment of roles for the Rho-specific guanine nucleotide dissociation inhibitor Ly-GDI in platelet function: a spatial systems approach.[Pubmed:28148498]
Am J Physiol Cell Physiol. 2017 Apr 1;312(4):C527-C536.
On activation at sites of vascular injury, platelets undergo morphological alterations essential to hemostasis via cytoskeletal reorganizations driven by the Rho GTPases Rac1, Cdc42, and RhoA. Here we investigate roles for Rho-specific guanine nucleotide dissociation inhibitor proteins (RhoGDIs) in platelet function. We find that platelets express two RhoGDI family members, RhoGDI and Ly-GDI. Whereas RhoGDI localizes throughout platelets in a granule-like manner, Ly-GDI shows an asymmetric, polarized localization that largely overlaps with Rac1 and Cdc42 as well as microtubules and protein kinase C (PKC) in platelets adherent to fibrinogen. Antibody interference and platelet spreading experiments suggest a specific role for Ly-GDI in platelet function. Intracellular signaling studies based on interactome and pathways analyses also support a regulatory role for Ly-GDI, which is phosphorylated at PKC substrate motifs in a PKC-dependent manner in response to the platelet collagen receptor glycoprotein (GP) VI-specific agonist collagen-related peptide. Additionally, PKC inhibition diffuses the polarized organization of Ly-GDI in spread platelets relative to its colocalization with Rac1 and Cdc42. Together, our results suggest a role for Ly-GDI in the localized regulation of Rho GTPases in platelets and hypothesize a link between the PKC and Rho GTPase signaling systems in platelet function.
Short-term dabigatran interruption before cardiac rhythm device implantation: multi-centre experience from the RE-LY trial.[Pubmed:28339794]
Europace. 2017 Oct 1;19(10):1630-1636.
Aims: Cardiac implantable electronic device (CIED) surgery is commonly performed in patients with atrial fibrillation (AF). The current analysis was undertaken to compare peri-operative anticoagulation management, bleeding, and thrombotic events in AF patients treated with dabigatran vs. warfarin. Methods and results: This study included 611 patients treated with dabigatran vs. warfarin who underwent CIED surgery during the RE-LY trial. Among 201 warfarin-treated patients, warfarin was interrupted a median of 144 (inter-quartile range, IQR: 120-216) h, and 37 (18.4%) patients underwent heparin bridging. In dabigatran-treated patients (216 on 110 mg bid and 194 on 150 mg bid), the duration of dabigatran interruption was a median of 96 (IQR: 61-158) h. Pocket hematomas occurred in 9 (2.20%) patients on dabigatran and 8 (3.98%) patients on warfarin (P = 0.218). The occurrence of pocket hematomas was lower with dabigatran compared with warfarin with heparin bridging (RD: -8.62%, 95% CI: -24.15 to - 0.51%, P = 0.034) but not when compared with warfarin with no bridging (P = 0.880). Ischemic stroke occurred in 2 (0.3%) patients; one in the warfarin group (without bridging) and one in the dabigatran 150 mg bid group (P = 0.735). Conclusion: In patients treated with dabigatran undergoing CIED surgery, interruption of dabigatran is associated with similar or lower incidence of pocket hematoma, when compared with warfarin interruption without or with heparin bridging, respectively. Whether uninterrupted dabigatran can reduce pocket hematoma or ischemic stroke remains to be evaluated.
Expression of serotonin7 receptor and coupling of ectopic receptors to protein kinase A and ionic currents in adrenocorticotropin-independent macronodular adrenal hyperplasia causing Cushing's syndrome.[Pubmed:16954157]
J Clin Endocrinol Metab. 2006 Nov;91(11):4578-86.
CONTEXT: In ACTH-independent macronodular adrenal hyperplasia (AIMAH) causing Cushing's syndrome, cortisol secretion is controlled by illegitimate membrane receptors. OBJECTIVE: The aim of the present study was to characterize the pharmacological properties and the transduction mechanisms of illegitimate receptors, i.e. receptors for serotonin (5-HT), gastric inhibitory polypeptide (GIP), and LH/human chorionic gonadotropin (hCG), expressed by AIMAH tissues to evaluate the role of ectopic receptors in the physiopathology of Cushing's syndrome. DESIGN: We used in vitro studies on cultured adrenal hyperplasia cells. SETTING: The setting was a university research laboratory. PATIENTS: AIMAH tissues (H1-H3) were removed from three patients previously screened for illegitimate receptors. MAIN OUTCOME MEASURE(S): The main outcome measures were steroidogenic and electrical activities of cultured adrenal hyperplasia cells. RESULTS: In vitro studies showed that the corticotropic effect of 5-HT was mediated by ectopic 5-HT7 receptors in H1 and H2. GIP and hCG stimulated cortisol production via activation of cAMP-dependent protein kinase A in H2. On the contrary, the protein kinase A inhibitor H-89 did not affect hCG-induced cortisol production in H3. Activation of 5-HT7 or GIP receptors enhanced T-type calcium current in H1 or H2 and H3, respectively. In addition, GIP reduced the amplitude of transient and sustained potassium currents in H2. Conversely, hCG did not modify T-type calcium current in H3. CONCLUSIONS: These data show that, besides their coupling to the cAMP pathway, illegitimate adrenal receptors can activate additional transduction mechanisms, including modulation of membrane channels.
Activation of 5-HT(7) receptor in rat glomerulosa cells is associated with an increase in adenylyl cyclase activity and calcium influx through T-type calcium channels.[Pubmed:11956157]
Endocrinology. 2002 May;143(5):1748-60.
Serotonin (5-HT) stimulates aldosterone secretion from the rat adrenal gland through 5-HT(7) receptors. The aim of the present study was to investigate the transduction mechanisms associated with activation of 5-HT(7) receptors in rat glomerulosa cells. The stimulatory effect of 5-HT on aldosterone secretion and cAMP formation was significantly reduced by the 5-HT(7) receptor antagonist LY 215840. Pretreatment of cells with the adenylyl cyclase inhibitor SQ 22536 or the PKA inhibitor H-89 markedly attenuated the effect of 5-HT on aldosterone secretion. Conversely, type 2 and 4 phosphodiesterase inhibitors potentiated the 5-HT-induced stimulation of aldosterone secretion. Administration of 5-HT in the vicinity of cultured glomerulosa cells induced a slowly developing and robust increase in cytosolic calcium concentration ([Ca(2+)](i)). The effect of 5-HT on [Ca(2+)](i) was suppressed by mibefradil, a T-type calcium channel blocker. Patch-clamp studies confirmed that 5-HT activated a T-type calcium current. Mibefradil also induced a dose-dependent inhibition of 5-HT-induced aldosterone secretion. The sequence of events associated with activation of 5-HT(7) receptors was investigated. The PKA inhibitor H-89 markedly attenuated both the [Ca(2+)](i) response and the activation of T-type calcium current induced by 5-HT. In contrast, reduction of the calcium concentration in the incubation medium did not affect 5-HT- induced cAMP formation. Preincubation of glomerulosa cells with cholera toxin abolished the stimulatory effect of 5-HT on aldosterone secretion, but pertussis toxin had no effect. Taken together, these data demonstrate that, in rat glomerulosa cells, activation of native 5-HT(7) receptors stimulates cAMP formation through a G(salpha) protein, which in turn provokes calcium influx through T-type calcium channels. Both the adenylyl cyclase/PKA pathway and the calcium influx are involved in 5-HT-induced aldosterone secretion.
LY215840, a high-affinity 5-HT7 receptor ligand, blocks serotonin-induced relaxation in canine coronary artery.[Pubmed:8667223]
J Pharmacol Exp Ther. 1996 Jun;277(3):1560-6.
The canine coronary artery possesses a smooth muscle relaxant serotonin (5-HT) receptor distinct from previously characterized 5-HT receptors. On the basis of the ability of LY53857 to block weakly coronary smooth muscle relaxation to 5-HT, we examined several structurally related ergolines in endothelium denuded rings of canine coronary artery precontracted with PGF2 alpha (10 microM). 5-HT (10 nM-100 microM)-induced relaxation was antagonized competitively by the ergoline esters LY53857 (-log KB = 6.5) and sergolexole (-log KB = 6.4) and by the ergoline amide amersergide, (-log KB = 6.7). In contrast to the relatively low affinity of these ergolines, LY215840, another ergoline amide, antagonized 5-HT-induced relaxation in a competitive manner with the highest affinity (-log KB = 8.3). This effect was independent of the 5-HT2 receptor affinity of these ergolines, because LY215840, LY53857, sergolexole and amesergide all possessed similar 5-HT2 receptor affinity. Further, all four ergolines possessed affinity for the human 5-HT7 receptor, and LY215840 had the highest 5-HT7 receptor affinity (Ki = 14.7 nM). Finally, in vascular smooth muscle under basal tone, LY215840 (1 microM) blocked the relaxant response to high concentrations of 5-HT and 5-MeOT without altering their contractile potency. LY215840 (1 microM) did not alter contraction to sumatriptan, an agent that lacks relaxant activity. In contrast, LY215840 (1 microM) markedly potentiated contraction to 5-carboxamidotryptamine, the most potent coronary relaxant agonist and the agonist with the highest 5-HT7 receptor affinity. The ability of LY215840 to block the relaxant 5-HT receptor in canine coronary artery may reflect its 5-HT7 receptor antagonist activity and make it a useful tool to probe the relationship between the 5-HT7 receptor and the coronary vasoactive properties of 5-HT.


