KH 7Selective soluble adenylyl cyclase inhibitor CAS# 330676-02-3 |
- PF-4981517
Catalog No.:BCC2270
CAS No.:1390637-82-7
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- Avasimibe
Catalog No.:BCC2274
CAS No.:166518-60-1
- Alizarin
Catalog No.:BCN3479
CAS No.:72-48-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 330676-02-3 | SDF | Download SDF |
| PubChem ID | 6843191 | Appearance | Powder |
| Formula | C17H15BrN4O2S | M.Wt | 419.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (238.49 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
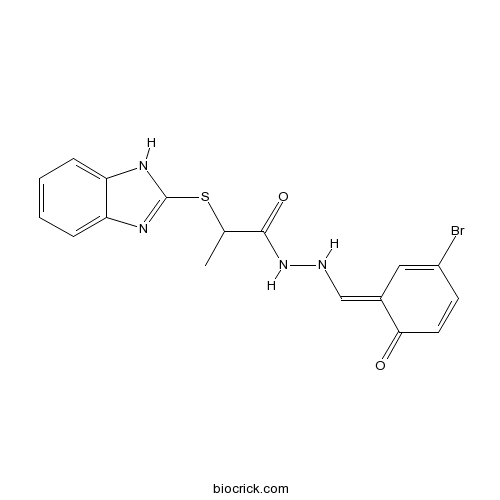
| Chemical Name | 2-(1H-benzimidazol-2-ylsulfanyl)-N'-[(3-bromo-6-oxocyclohexa-2,4-dien-1-ylidene)methyl]propanehydrazide | ||
| SMILES | CC(C(=O)NNC=C1C=C(C=CC1=O)Br)SC2=NC3=CC=CC=C3N2 | ||
| Standard InChIKey | GEHVZUUHMWNMAY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H15BrN4O2S/c1-10(25-17-20-13-4-2-3-5-14(13)21-17)16(24)22-19-9-11-8-12(18)6-7-15(11)23/h2-10,19H,1H3,(H,20,21)(H,22,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective soluble adenylyl cyclase (sAC) inhibitor (IC50 = 3 - 10 μM in vivo). Inert towards transmembrane adenylyl cyclase (tmAC) in vitro and in whole cells at concentrations up to 300 μM. Blocks synthesis of cAMP and displays an antiapoptotic effect at concentrations of 1 - 100 μM. |

KH 7 Dilution Calculator

KH 7 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3849 mL | 11.9246 mL | 23.8493 mL | 47.6985 mL | 59.6232 mL |
| 5 mM | 0.477 mL | 2.3849 mL | 4.7699 mL | 9.5397 mL | 11.9246 mL |
| 10 mM | 0.2385 mL | 1.1925 mL | 2.3849 mL | 4.7699 mL | 5.9623 mL |
| 50 mM | 0.0477 mL | 0.2385 mL | 0.477 mL | 0.954 mL | 1.1925 mL |
| 100 mM | 0.0238 mL | 0.1192 mL | 0.2385 mL | 0.477 mL | 0.5962 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- HCTU
Catalog No.:BCC2818
CAS No.:330645-87-9
- TCTU
Catalog No.:BCC2689
CAS No.:330641-16-2
- Peramivir
Catalog No.:BCC1846
CAS No.:330600-85-6
- H-Orn(Z)-OH
Catalog No.:BCC3003
CAS No.:3304-51-6
- Aloe-emodin-8-O-beta-D-glucopyranoside
Catalog No.:BCN1456
CAS No.:33037-46-6
- Boc-β-Ala-OH
Catalog No.:BCC3051
CAS No.:3303-84-2
- TC HSD 21
Catalog No.:BCC6228
CAS No.:330203-01-5
- SU6656
Catalog No.:BCC6392
CAS No.:330161-87-0
- Cyanidin-3-O-sambubioside chloride
Catalog No.:BCN3150
CAS No.:33012-73-6
- ent-16beta,17-Dihydroxy-19-kauranoic acid
Catalog No.:BCN1457
CAS No.:3301-61-9
- Kumatakenin
Catalog No.:BCN5252
CAS No.:3301-49-3
- Nicarbazin
Catalog No.:BCC9101
CAS No.:330-95-0
- Paclitaxel
Catalog No.:BCN4650
CAS No.:33069-62-4
- Avanafil
Catalog No.:BCC2288
CAS No.:330784-47-9
- PCI 29732
Catalog No.:BCC4100
CAS No.:330786-25-9
- MRT 10
Catalog No.:BCC7950
CAS No.:330829-30-6
- Amitraz
Catalog No.:BCC8816
CAS No.:33089-61-1
- Betrixaban
Catalog No.:BCC5118
CAS No.:330942-05-7
- AS 1269574
Catalog No.:BCC7878
CAS No.:330981-72-1
- Caffeic acid
Catalog No.:BCN5979
CAS No.:331-39-5
- IQ 1
Catalog No.:BCC7965
CAS No.:331001-62-8
- PT 1
Catalog No.:BCC7846
CAS No.:331002-70-1
- Stephavanine
Catalog No.:BCN5253
CAS No.:33116-33-5
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
Crystal structure of the first KH domain of human poly(C)-binding protein-2 in complex with a C-rich strand of human telomeric DNA at 1.7 A.[Pubmed:16186123]
J Biol Chem. 2005 Nov 18;280(46):38823-30.
Recognition of poly(C) DNA and RNA sequences in mammalian cells is achieved by a subfamily of the KH (hnRNP K homology) domain-containing proteins known as poly(C)-binding proteins (PCBPs). To reveal the molecular basis of poly(C) sequence recognition, we have determined the crystal structure, at 1.7-A resolution, of PCBP2 KH1 in complex with a 7-nucleotide DNA sequence (5'-AACCCTA-3') corresponding to one repeat of the human C-rich strand telomeric DNA. The protein-DNA interaction is mediated by the combination of several stabilizing forces including hydrogen bonding, electrostatic interactions, van der Waals contacts, and shape complementarities. Specific recognition of the three cytosine residues is realized by a dense network of hydrogen bonds involving the side chains of two conserved lysines and one glutamic acid. The co-crystal structure also reveals a protein-protein dimerization interface of PCBP2 KH1 located on the opposite side of the protein from the DNA binding groove. Numerous stabilizing protein-protein interactions, including hydrophobic contacts, stacking of aromatic side chains, and a large number of hydrogen bonds, indicate that the protein-protein interaction interface is most likely genuine. Interaction of PCBP2 KH1 with the C-rich strand of human telomeric DNA suggests that PCBPs may participate in mechanisms involved in the regulation of telomere/telomerase functions.
Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells.[Pubmed:19336406]
J Biol Chem. 2009 May 29;284(22):14760-8.
The cAMP signaling pathway plays an essential role in modulating the apoptotic response to various stress stimuli. Until now, it was attributed exclusively to the activity of the G-protein-responsive transmembrane adenylyl cyclase. In addition to transmembrane AC, mammalian cells possess a second source of cAMP, the ubiquitously expressed soluble adenylyl cyclase (sAC). However, the role of this cyclase in apoptosis was unknown. A mitochondrial localization of this cyclase has recently been demonstrated, which led us to the hypothesis that sAC may play a role in apoptosis through modulation of mitochondria-dependent apoptosis. To prove this hypothesis, apoptosis was induced by simulated in vitro ischemia or by acidosis, which is an important component of ischemia. Suppression of sAC activity with the selective inhibitor KH7 or sAC knockdown by small interfering RNA transfection abolished endothelial apoptosis. Furthermore, pharmacological inhibition or knockdown of protein kinase A, an important cAMP target, demonstrated a significant anti-apoptotic effect. Analysis of the underlying mechanisms revealed (i) the translocation of sAC to mitochondria under acidic stress and (ii) activation of the mitochondrial pathway of apoptosis, i.e. cytochrome c release and caspase-9 cleavage. sAC inhibition or knockdown abolished the activation of the mitochondrial pathway of apoptosis. Analysis of mitochondrial co-localization of Bcl-2 family proteins demonstrated sAC- and protein kinase A-dependent translocation of Bax to mitochondria. Taken together, these results suggest the important role of sAC in modulating the mitochondria-dependent pathway of apoptosis in endothelial cells.
Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1.[Pubmed:16627466]
J Biol Chem. 2006 Jun 23;281(25):17253-8.
Nerve growth factor (NGF) and the ubiquitous second messenger cyclic AMP (cAMP) are both implicated in neuronal differentiation. Multiple studies indicate that NGF signals to at least a subset of its targets via cAMP, but the link between NGF and cAMP has remained elusive. Here, we have described the use of small molecule inhibitors to differentiate between the two known sources of cAMP in mammalian cells, bicarbonate- and calcium-responsive soluble adenylyl cyclase (sAC) and G protein-regulated transmembrane adenylyl cyclases. These inhibitors, along with sAC-specific small interfering RNA, reveal that sAC is uniquely responsible for the NGF-elicited rise in cAMP and is essential for the NGF-induced activation of the small G protein Rap1 in PC12 cells. In contrast and as expected, transmembrane adenylyl cyclase-generated cAMP is responsible for Rap1 activation by the G protein-coupled receptor ligand PACAP (pituitary adenylyl cyclase-activating peptide). These results identify sAC as a mediator of NGF signaling and reveal the existence of distinct pathways leading to cAMP-dependent signal transduction.


