Juncuenin BCAS# 1161681-20-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1161681-20-4 | SDF | Download SDF |
| PubChem ID | 44178766 | Appearance | Powder |
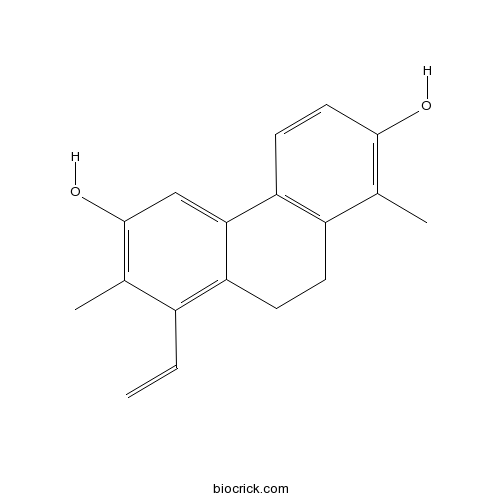
| Formula | C18H18O2 | M.Wt | 266.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 8-ethenyl-1,7-dimethyl-9,10-dihydrophenanthrene-2,6-diol | ||
| SMILES | CC1=C(C=CC2=C1CCC3=C(C(=C(C=C32)O)C)C=C)O | ||
| Standard InChIKey | FJDKWDFWAXRBGQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H18O2/c1-4-12-10(2)18(20)9-16-14(12)6-5-13-11(3)17(19)8-7-15(13)16/h4,7-9,19-20H,1,5-6H2,2-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Juncuenin B Dilution Calculator

Juncuenin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7552 mL | 18.7758 mL | 37.5516 mL | 75.1033 mL | 93.8791 mL |
| 5 mM | 0.751 mL | 3.7552 mL | 7.5103 mL | 15.0207 mL | 18.7758 mL |
| 10 mM | 0.3755 mL | 1.8776 mL | 3.7552 mL | 7.5103 mL | 9.3879 mL |
| 50 mM | 0.0751 mL | 0.3755 mL | 0.751 mL | 1.5021 mL | 1.8776 mL |
| 100 mM | 0.0376 mL | 0.1878 mL | 0.3755 mL | 0.751 mL | 0.9388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Dalbergiphenol
Catalog No.:BCN9262
CAS No.:82358-44-9
- 9-Oxooctadeca-10,12-dienoic acid
Catalog No.:BCN9261
CAS No.:54232-58-5
- Gancaonin O
Catalog No.:BCN9260
CAS No.:129145-53-5
- Jasminoside
Catalog No.:BCN9259
CAS No.:82451-18-1
- threo-Guaiacylglycerol β-coniferyl ether
Catalog No.:BCN9258
CAS No.:168393-18-8
- Matairesinol monoglucoside
Catalog No.:BCN9257
CAS No.:34446-06-5
- 5-O-Methyllatifolin
Catalog No.:BCN9256
CAS No.:18525-14-9
- Cinchonain Ib
Catalog No.:BCN9255
CAS No.:85022-69-1
- 16,23-Oxidoalisol B
Catalog No.:BCN9254
CAS No.:169326-06-1
- 2-Methoxy-1,6-dimethyl-5-vinyl-9,10-dihydrophenanthren-7-ol
Catalog No.:BCN9253
CAS No.:2266586-31-4
- Jinflexin A
Catalog No.:BCN9252
CAS No.:2055155-75-2
- 1,6-Dimethyl-5-vinyl-9,10-dihydrophenanthren-2-ol
Catalog No.:BCN9251
CAS No.:745056-83-1
- Juncuenin A
Catalog No.:BCN9264
CAS No.:1161681-18-0
- Sepiumol E
Catalog No.:BCN9265
CAS No.:2412027-09-7
- 4-Demethyltraxillaside
Catalog No.:BCN9266
CAS No.:1691201-82-7
- Tatsinine
Catalog No.:BCN9267
CAS No.:90038-21-4
- Platyphyllonol 5-O-β-D-xylopyranoside
Catalog No.:BCN9268
CAS No.:288141-04-8
- Cavaol E
Catalog No.:BCN9269
CAS No.:1233044-20-6
- Hedyotol D
Catalog No.:BCN9270
CAS No.:97465-80-0
- Pregn-5-ene-3β,17α,20S-triol
Catalog No.:BCN9271
CAS No.:903-67-3
- 6-Methoxyspirotryprostatin B
Catalog No.:BCN9272
CAS No.:1031727-28-2
- 3-(3-Hydroxybutyl)phenol
Catalog No.:BCN9273
CAS No.:854464-95-2
- Phoyunbene B
Catalog No.:BCN9274
CAS No.:886747-62-2
- Effususol A
Catalog No.:BCN9275
CAS No.:1869082-58-5
Anxiolytic effect of a novel 9,10-dihydrophenanthrene, juncuenin H, is associated with metabolic changes in cortical serotonin/dopamine levels in mice.[Pubmed:30825572]
Fitoterapia. 2019 Apr;134:165-171.
Two novel phenanthrenoids, juncuenin H (1) and diJuncuenin B (2), together with eight known phenanthrenoids, effusol (3), dehydroeffusol (4), juncusol (5), dehydrojuncusol (6), Juncuenin B (7), dehydroJuncuenin B (8), juncuenin A (9), and dehydrojuncuenin A (10), were isolated from the underground parts of Juncus setchuenensis. The structures of the compounds were determined by 1D and 2D NMR and mass spectroscopy. The anxiolytic activities of compounds 1, 6, 9, and 10 were evaluated. In order to explore the mechanisms underlying their anxiolytic activities, the levels of serotonin (5-HT), dopamine (DA), and their metabolites in the cerebral cortex and hippocampus of mice treated with compound 1 were determined by quantitative mass spectrometry. The mice treated with compound 1 had significantly lower levels of 5-HT, 3-methoxytyramine (3-MT), 5-hydroxyindole-3-acetic acid (5-HIAA), homovanillic acid (HVA), and 3, 4-dihydroxyphenylacetic acid (DOPAC) in the cerebral cortex than those of the vehicle control-treated mice. The levels of HVA and 5-HIAA in the hippocampus were also significantly lower in the mice treated with compound 1 than in the control group mice. These results suggest that the metabolic changes, reflected in the levels of DA and/or 5-HT, may contribute to the anxiolytic activity of the phenanthrenoids studied herein.
Screening of Luzula species native to the Carpathian Basin for anti-inflammatory activity and bioactivity-guided isolation of compounds from Luzula luzuloides (Lam.) Dandy & Wilmott.[Pubmed:27940118]
Fitoterapia. 2017 Jan;116:131-138.
The present study focused on the anti-inflammatory screening of Luzula species native to the Carpathian Basin and bioactivity-guided isolation of compounds of Luzula luzuloides (Lam.) Dandy & Wilmott. The anti-inflammatory properties of extracts with different polarity prepared from Luzula species were determined. Among them, the CH2Cl2-soluble fraction of L. luzuloides possessed strong inhibitory effects on superoxide anion generation (99.39+/-0.37%) and elastase release (114.22+/-3.13%) in fMLP/CB-induced human neutrophils at concentration of 10mug/mL. From this fraction, six compounds (1-6) were isolated by the combination of different chromatographic methods. The structures of the compounds were determined by means of MS, 1D and 2D NMR spectroscopy. The results allowed the identification of the new 1,6-dihydroxy-2-keto-1,7-dimethyl-8-vinyl-1,2-dihydrophenanthrene (1) from the plant, named luzulin A. Chiral HPLC and HPLC-ECD analysis revealed that 1 possesses low enantiomeric excess and TDDFT-ECD calculations afforded the configurational assignment of the separated enantiomers. Three known phenanthrenes [Juncuenin B (2), dehydroJuncuenin B (3) and juncusol (4)] and two flavonoids [apigenin (5) and luteolin (6)] were also isolated. The anti-inflammatory activity of the isolated compounds was tested and IC50 values were determined. This was the first time that phenanthrenes were detected in a Luzula species. The oxidative transformation of Juncuenin B (3) led to the isolation of its possible biometabolites, namely luzulin A (1), dehydroJuncuenin B (4), and juncuenin D (7). The isolated compounds (1-4) confirm that besides flavonoids, phenanthrenes could also serve as chemotaxonomic markers for Luzula species and prove the close relationship of Juncus and Luzula genus.
Phenanthrenes from Juncus inflexus with Antimicrobial Activity against Methicillin-Resistant Staphylococcus aureus.[Pubmed:27808510]
J Nat Prod. 2016 Nov 23;79(11):2814-2823.
The present study has focused on an investigation of the antibacterial effects of Juncus inflexus and the isolation and identification of its active compounds. Eleven phenanthrenes were isolated from a methanolic extract of the roots. Four compounds (jinflexins A-D, 1-4) are new natural products, while seven phenanthrenes [juncuenins A (5), B (6), and D (8), juncusol (7), dehydrojuncuenins A (9) and B (11), and dehydrojuncusol (10)] were isolated for the first time from the plant. Jinflexin D (4) is a dimer with an unprecedented heptacyclic ring system. The absolute configurations of the new compounds were determined by TDDFT-ECD calculations, and their enantiomeric purity was checked by chiral HPLC analysis. Extracts of different polarity (n-hexane, dichloromethane, and ethyl acetate) were evaluated for their antimicrobial effects against methicillin-resistant Staphylococcus aureus, extended-spectrum beta-lactamase (ESBL)-producing Citrobacter freundii, Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, multiresistant Acinetobacter baumannii, and Pseudomonas aeruginosa. The MIC values of the isolated compounds were determined by a microdilution method. Jinflexin B (2), juncusol (7), juncuenin D (8), and dehydroJuncuenin B (11) showed significant activity (MIC value range 12.5-100 mug/mL) against MRSA strains.
Antibacterial screening of Juncaceae species native to the Carpathian Basin against resistant strains and LC-MS investigation of phenanthrenes responsible for the effect.[Pubmed:27702667]
Fitoterapia. 2016 Dec;115:69-73.
The main objective of this project was to investigate the antibacterial activity of 19 species (Juncus acutus, J. alpinoarticulatus, J. articulatus, J. compressus, J. conglomeratus, J. effusus, J. filiformis, J. gerardii, J. inflexus, J. maritimus, J. monanthos, J. squarrosus, J. tenuis, J. trifidus, Luzula campestris, L. forsteri, L. luzuloides, L. sudetica and L. sylvatica) belonging to the family Juncaceae against methicillin-resistant S. aureus (MRSA), extended-spectrum beta-lactamase (ESBL)-producing C. freundii, E. coli, E. cloacae, K. pneumoniae, and multiresistant A. baumannii and P. aeruginosa. Antibacterial susceptibilities were screened for inhibitory zones and MIC values determined by microdilution method. Among the tested extracts (n=96) 16 extracts prepared from Juncus species and 3 extracts from Luzula species showed mild to strong inhibitory activities against MRSA strains (inhibition zones=6.7mm-14.6mm; MIC values 9.75-156mug/mL). It can be concluded that Juncus and Luzula species demonstrated promising anti-MRSA effect, and J. maritimus, J. tenuis and J. gerardii considered worthy of activity-guided phytochemical investigations. The main bioactive constituents of Juncaceae species are phenanthrenes. Four phenanthrenes [juncuenin D (1), juncusol (2), dehydroJuncuenin B (3), and jinflexin B (4)] isolated previously from J. inflexus with anti-MRSA activity were investigated by LC-MS in extracts proved to be active in antimicrobial test.
[Phenanthrenes from aerial part of Juncus setchuensis with anxiolyticactivity].[Pubmed:28875672]
Zhongguo Zhong Yao Za Zhi. 2016 Mar;41(6):1070-1074.
Ten phenanthrenes, two organic acids, one organic acid ester and one flavonoid were isolated from the aerial part of Juncus setchuensis by various chromatographic techniques usingsilica gel, polyamide, Sephadex LH-20 as solid phases, and preparative HPLC. Their structures were identified by MS and NMR spectroscopic data as effusol(1), juncusol(2), juncuenin D(3), dehydroeffusol(4), dehydrojuncusol(5), Juncuenin B(6),dehydroJuncuenin B(7), 2-methoxyl-7-hydroxyl-1-methyl-5-vinyl phenanthrene(8), 2-hydroxyl-7-carboxy-1-methyl-5-vinyl-9,10-dihydrophenanthrene(9), 2-hydroxyl-7-carboxyl-1-methyl-5-vinylphenanthrene(10), luteolin(11), vanillic acid(12), daphnetin(13), p-coumaric acid(14), respectively. Compound 13 was isolated from the genus Juncus for the first time and compounds 5, 8-12 were isolated from J. setchuensis for the first time. The elevated plus-maze(EPM) was used to evaluate the anxiolytic activity of compounds 6 and 7. Compound 6 at 5 mg*kg(-)(1) and 10 mg*kg(-)(1) showed anxiolytic activity as well as compound 7 at 10 mg*kg(-)(1) and 20 mg*kg(-)(1).
Chemical constituents isolated from Juncus effusus induce cytotoxicity in HT22 cells.[Pubmed:25794817]
J Nat Med. 2015 Jul;69(3):421-6.
Effususol A (1), a new 9,10-dihydrophenanthrene, has been isolated from the medullae of Juncus effusus along with ten known compounds, effusol (2), dehydroeffusol (3), juncusol (4), dehydrojuncusol (5), Juncuenin B (6), dehydroJuncuenin B (7), juncuenin D (8), luteolin (9), luteolin 5-methyl ether (10), and 4-hydroxy-2,3-dimethyl-2-nonen-4-olide (11). The structure of 1 was elucidated on the basis of spectroscopic data. 2, 4, 6, 7, and 8 have induced caspase-3-mediated cytotoxicity in HT22 cells.


