JasminosideCAS# 82451-18-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 82451-18-1 | SDF | Download SDF |
| PubChem ID | 138113058 | Appearance | Powder |
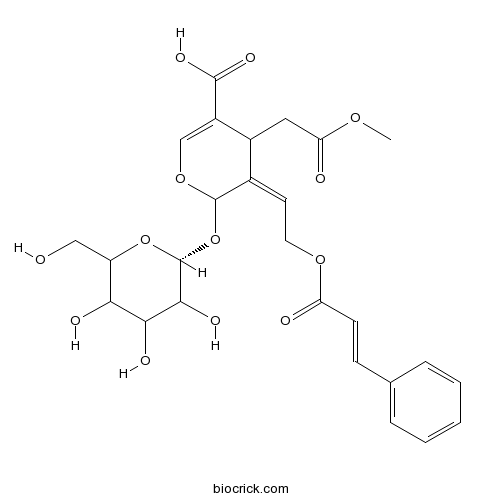
| Formula | C26H30O13 | M.Wt | 550.5 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-(2-methoxy-2-oxoethyl)-5-[2-(3-phenylprop-2-enoyloxy)ethylidene]-6-[(2R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4H-pyran-3-carboxylic acid | ||
| SMILES | COC(=O)CC1C(=COC(C1=CCOC(=O)C=CC2=CC=CC=C2)OC3C(C(C(C(O3)CO)O)O)O)C(=O)O | ||
| Standard InChIKey | JGHUOJAZXGSFRI-XSGNIEOLSA-N | ||
| Standard InChI | InChI=1S/C26H30O13/c1-35-20(29)11-16-15(9-10-36-19(28)8-7-14-5-3-2-4-6-14)25(37-13-17(16)24(33)34)39-26-23(32)22(31)21(30)18(12-27)38-26/h2-9,13,16,18,21-23,25-27,30-32H,10-12H2,1H3,(H,33,34)/t16?,18?,21?,22?,23?,25?,26-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Jasminoside Dilution Calculator

Jasminoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8165 mL | 9.0827 mL | 18.1653 mL | 36.3306 mL | 45.4133 mL |
| 5 mM | 0.3633 mL | 1.8165 mL | 3.6331 mL | 7.2661 mL | 9.0827 mL |
| 10 mM | 0.1817 mL | 0.9083 mL | 1.8165 mL | 3.6331 mL | 4.5413 mL |
| 50 mM | 0.0363 mL | 0.1817 mL | 0.3633 mL | 0.7266 mL | 0.9083 mL |
| 100 mM | 0.0182 mL | 0.0908 mL | 0.1817 mL | 0.3633 mL | 0.4541 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- threo-Guaiacylglycerol β-coniferyl ether
Catalog No.:BCN9258
CAS No.:168393-18-8
- Matairesinol monoglucoside
Catalog No.:BCN9257
CAS No.:34446-06-5
- 5-O-Methyllatifolin
Catalog No.:BCN9256
CAS No.:18525-14-9
- Cinchonain Ib
Catalog No.:BCN9255
CAS No.:85022-69-1
- 16,23-Oxidoalisol B
Catalog No.:BCN9254
CAS No.:169326-06-1
- 2-Methoxy-1,6-dimethyl-5-vinyl-9,10-dihydrophenanthren-7-ol
Catalog No.:BCN9253
CAS No.:2266586-31-4
- Jinflexin A
Catalog No.:BCN9252
CAS No.:2055155-75-2
- 1,6-Dimethyl-5-vinyl-9,10-dihydrophenanthren-2-ol
Catalog No.:BCN9251
CAS No.:745056-83-1
- Dauricumine
Catalog No.:BCN9250
CAS No.:345641-00-1
- Murrayanine
Catalog No.:BCN9249
CAS No.:723-97-7
- Dictysine
Catalog No.:BCN9248
CAS No.:67256-05-7
- Qingyangshengenin 3-O-β-D-cymaropyranosyl-(1→4)-β-D-digitoxopyranoside
Catalog No.:BCN9247
CAS No.:1186628-87-4
- Gancaonin O
Catalog No.:BCN9260
CAS No.:129145-53-5
- 9-Oxooctadeca-10,12-dienoic acid
Catalog No.:BCN9261
CAS No.:54232-58-5
- (+)-Dalbergiphenol
Catalog No.:BCN9262
CAS No.:82358-44-9
- Juncuenin B
Catalog No.:BCN9263
CAS No.:1161681-20-4
- Juncuenin A
Catalog No.:BCN9264
CAS No.:1161681-18-0
- Sepiumol E
Catalog No.:BCN9265
CAS No.:2412027-09-7
- 4-Demethyltraxillaside
Catalog No.:BCN9266
CAS No.:1691201-82-7
- Tatsinine
Catalog No.:BCN9267
CAS No.:90038-21-4
- Platyphyllonol 5-O-β-D-xylopyranoside
Catalog No.:BCN9268
CAS No.:288141-04-8
- Cavaol E
Catalog No.:BCN9269
CAS No.:1233044-20-6
- Hedyotol D
Catalog No.:BCN9270
CAS No.:97465-80-0
- Pregn-5-ene-3β,17α,20S-triol
Catalog No.:BCN9271
CAS No.:903-67-3
A two-step ultra-high-performance liquid chromatography-quadrupole/time of flight mass spectrometry with mass defect filtering method for rapid identification of analogues from known components of different chemical structure types in Fructus Gardeniae-Fructus Forsythiae herb pair extract and in rat's blood.[Pubmed:29861306]
J Chromatogr A. 2018 Aug 17;1563:99-123.
Fructus Gardeniae-Fructus Forsythiae herb pair is an herbal formula used extensively to treat inflammation and fever, but few systematic identification studies of the bioactive components have been reported. Herein, the unknown analogues in the first-step screening were rapidly identified from representative compounds in different structure types (geniposide as iridoid type, crocetin as crocetin type, Jasminoside B as monocyclic monoterpene type, oleanolic acid as saponin type, 3-caffeoylquinic acid as organic acid type, forsythoside A as phenylethanoid type, phillyrin as lignan type and quercetin 3-rutinoside as flavonoid type) by UPLC-Q-Tof/MS combined with mass defect filtering (MDF), and further confirmed with reference standards and published literatures. Similarly, in the second step, other unknown components were rapidly discovered from the compounds identified in the first step by MDF. Using the two-step screening method, a total of 58 components were characterized in Fructus Gardeniae-Fructus Forsythiae (FG-FF) decoction. In rat's blood, 36 compounds in extract and 16 metabolites were unambiguously or tentatively identified. Besides, we found the principal metabolites were glucuronide conjugates, with the glucuronide conjugates of caffeic acid, quercetin and kaempferol confirmed as caffeic acid 3-glucuronide, quercetin 3-glucuronide and kaempferol 3-glucuronide by reference standards, respectively. Additionally, most of them bound more strongly to human serum albumin than their respective prototypes, predicted by Molecular Docking and Simulation, indicating that they had lower blood clearance in vivo and possibly more contribution to pharmacological effects. This study developed a novel two-step screening method in addressing how to comprehensively screen components in herbal medicine by UPLC-Q-Tof/MS with MDF.
[Study on chemical constituents of Gardenia jasminoides(III)].[Pubmed:25566656]
Zhong Yao Cai. 2014 Jul;37(7):1196-9.
OBJECTIVE: To investigate the chemical constituents of Gardenia jasminoides fruits. METHODS: Various column chromatography were used in the isolation and purification, and physiochemical constant determination and spectral analysis were adopted to determine the chemical structures. RESULTS: Twelve compounds were isolated from Gardenia jasminoides including Jasminoside I (1), gardenoside (2), gardaloside (3), 3-hydroxy-urs-12-ene-11-ketone(4), 5, 4'-dihydroxyl-7, 3', 5'-trimethoxyflavone (5), 5, 7, 3', 4', 5'-pentamethoxyflavone(6), 3, 5, 6, 4'-tetrahydroxy-3', 5'-dimethoxyflavone (7), shikimic acid (8), 1, 2, 4-benzenetriol (9), 3, 4-dimethoxy-benzoic acid (10), dibutyl phthalate (11) and diisobutyl phthalate (12). CONCLUSION: Compounds 4 - 7 and 9 -10 were isolated from this plant for the first time.
[Study on the chemical components of Gardenia jasminoides (II)].[Pubmed:24010321]
Zhong Yao Cai. 2013 Mar;36(3):401-3.
OBJECTIVE: To investigate the chemical components of Gardenia jasminoides. METHODS: Various column chromatography were used in the isolation and purification, and physiochemical constant determination and spectral analysis were adopted to idenitify the chemical structures. RESULTS: Ten compounds were isolated and identified as Jasminoside A(1), epiJasminoside A(2), 6-O-methylscandoside methyl ester (3), 6-O-methyldeacetylasperulosidic acid methyl ester (4), gardenoside (5), phenylmethol (6), 4-hydroxy-phenylmethol-O-beta-D-glucopyranosyl- (1-->6) -beta-D-glucopyranoside (7), 3,4-dihydroxy-phenylmethol-O-beta-D-glucopyranosyl-(1-6)-beta-D-glucopyranoside (8), 3-hydroxy4-methoxy-phenylmethol-O-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyran oside (9), 3-hydroxy-4-methoxyphenylmethol-O-beta-D-glucopyranoside (10). CONCLUSION: Compounds 6 -10 are isolated from this plant for the first time.
Chemical constituents from the fruit of Gardenia jasminoides and their inhibitory effects on nitric oxide production.[Pubmed:23305920]
Bioorg Med Chem Lett. 2013 Feb 15;23(4):1127-31.
Three new iridoid glycosides, 6"-O-trans-caffeoylgenipin gentiobioside (1), genipin 1-O-beta-D-apiofuranosyl (1-->6)-beta-D-glucopyranoside (2), genipin 1-O-alpha-D-xylopyranosyl (1-->6)-beta-D-glucopyranoside (3), three new monocyclic monoterpenoids, Jasminoside R (4), Jasminoside S (5), Jasminoside T (6), together with nine known iridoid glycosides (7-15) and three crocetin glycosides (16-18), were isolated from the fruit of Gardenia jasminoides. Their chemical structures were established mainly by 1D and 2D NMR techniques and mass spectrometry. Inhibitory effects of the isolated compounds on nitric oxide production in lipopolysaccaride-activated macrophages were evaluated. Compounds 8 and 18 showed strong inhibitory activity on NO production with IC(50) values of 11.14+/-0.67 and 5.99+/-0.54 muM, respectively.
New spirostanol glycosides from Solanum nigrum and S. jasminoides.[Pubmed:22388971]
J Nat Med. 2012 Oct;66(4):658-63.
A new characteristic steroidal glycoside possessing a hydroxyl group at C-23, inunigroside A (1), was isolated from the withered berries of Solanum nigrum L. On the basis of spectroscopic analysis, the structure of 1 was characterized as (5alpha,22S,23S,25R)-3beta,23-dihydroxyspirostane 3-O-beta-lycotetraoside. Next, a major steroidal sapogenol, (22R, 25S)-3beta,15alpha-dihydroxy-spirost-5-ene (3), was obtained from the acid hydrolysate of the methanolic extract of the aerial parts of Solanum jasminoides L. A new bisdesmoside, 3-O-beta-D-glucopyranosyl-(1-->4)-beta-D-glucopyranosyl-(1-->4)-beta-D-glucopyran osyl (22R,25S)-3beta,15alpha-dihydroxyspirost-5-ene 15-O-alpha-L-rhamnopyranoside (4), named Jasminoside A, was isolated from the methanolic extract of S. jasminoides.
Pyronane monoterpenoids from the fruit of Gardenia jasminoides.[Pubmed:18505286]
J Nat Prod. 2008 Jun;71(6):995-9.
Five new pyronane-type monocyclic monoterpenoids, jasminodiol (1), Jasminoside H (6), 6'-O-sinapoylJasminoside A (7), 6'-O-sinapoylJasminoside C (8), and Jasminoside I (9), together with four known analogues, were isolated from the fruit of Gardenia jasminoides. The structures of the new metabolites were characterized using spectroscopic data, and the absolute configurations of 1, 6, and 7 were established using circular dichroism (CD) analysis. Compound 1 showed tyrosinase inhibitory activity (IC 50 2.2 mM).
Immunosuppressive iridoids from the fruits of Gardenia jasminoides.[Pubmed:16309325]
J Nat Prod. 2005 Nov;68(11):1683-5.
A new iridoid, gardaloside (1), and a new safranal-type monoterpene, Jasminoside G (2), together with 10 known compounds including nine iridoids and a second safranal-type monoterpene, were isolated from the fruits of Gardenia jasminoides. The structures of 1 and 2 were established on the basis of spectroscopic evidence. Of these compounds, geniposide (3), 6alpha-hydroxygeniposide (5), ixoroside (7), and shanzhiside (8) showed significant inhibition of IL-2 secretion by phorbol myristate acetate and anti-CD28 monoclonal antibody co-stimulated activation of human peripheral blood T cells.
Studies of the constituents of Gardenia species. II. Terpenoids from Gardeniae Fructus.[Pubmed:10823717]
Chem Pharm Bull (Tokyo). 2000 May;48(5):746-8.
Four new terpenoids, gardenate A (1), 2-hydroxyethyl gardenamide A (2), (1R,7R,8S,10R)-7,8,11-trihydroxyguai-4-en-3-one 8-O-beta-D-glucopyranoside (3) and Jasminoside F (4), were isolated from Gardeniae Fructus. Their structures were established on the basis of spectral analysis.
New secoiridoid glucosides from Jasminum lanceolarium.[Pubmed:17252492]
Planta Med. 1996 Dec;62(6):515-8.
Two new secoiridoid glucosides, the trans-P-coumaroyl and trans-feruloyl esters of 10-hydroxyoleoside, jaslanceosides A (2) and B (3), were isolated from the leaves and stems of Jasminum lanceolarium (Oleaceae) in addition to Jasminoside (1) and 10-hydroxyoleoside dimethyl ester (8). The structures of these compounds have been elucidated on the basis of spectral and chemical methods.
Jasamplexosides A, B and C: Novel Dimeric and Trimeric Secoiridoid Glucosides from Jasminum amplexicaule.[Pubmed:17226520]
Planta Med. 1992 Dec;58(6):552-5.
Three new secoiridoid glucosides, jasamplexosides A, B and C, were isolated from the crude drug "Niu du teng", the leaves and stems of JASMINUM AMPLEXICAULE, together with the known secoiridoid glucosides, 10-hydroxyligstroside and Jasminoside. Their structural elucidation by spectroscopic studies is described.


