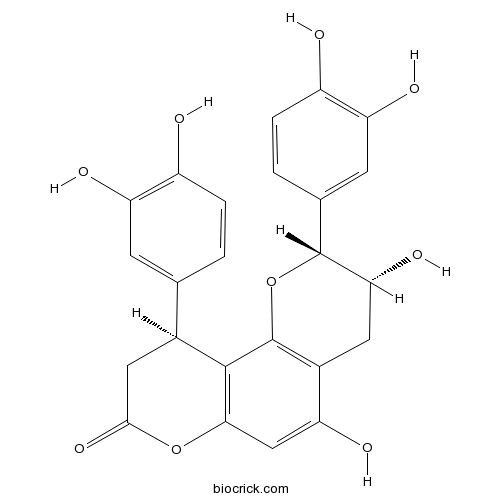
Cinchonain IbCAS# 85022-69-1 |
- Cinchonain Ia
Catalog No.:BCN9243
CAS No.:85081-24-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 85022-69-1 | SDF | Download SDF |
| PubChem ID | 10456516 | Appearance | Powder |
| Formula | C24H20O9 | M.Wt | 452.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R,10S)-2,10-bis(3,4-dihydroxyphenyl)-3,5-dihydroxy-3,4,9,10-tetrahydro-2H-pyrano[2,3-f]chromen-8-one | ||
| SMILES | C1C(C(OC2=C1C(=CC3=C2C(CC(=O)O3)C4=CC(=C(C=C4)O)O)O)C5=CC(=C(C=C5)O)O)O | ||
| Standard InChIKey | LKCOZWLUAKSRQM-IBUUURQNSA-N | ||
| Standard InChI | InChI=1S/C24H20O9/c25-14-3-1-10(5-17(14)28)12-8-21(31)32-20-9-16(27)13-7-19(30)23(33-24(13)22(12)20)11-2-4-15(26)18(29)6-11/h1-6,9,12,19,23,25-30H,7-8H2/t12-,19+,23+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cinchonain Ib Dilution Calculator

Cinchonain Ib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2104 mL | 11.0522 mL | 22.1043 mL | 44.2087 mL | 55.2608 mL |
| 5 mM | 0.4421 mL | 2.2104 mL | 4.4209 mL | 8.8417 mL | 11.0522 mL |
| 10 mM | 0.221 mL | 1.1052 mL | 2.2104 mL | 4.4209 mL | 5.5261 mL |
| 50 mM | 0.0442 mL | 0.221 mL | 0.4421 mL | 0.8842 mL | 1.1052 mL |
| 100 mM | 0.0221 mL | 0.1105 mL | 0.221 mL | 0.4421 mL | 0.5526 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 16,23-Oxidoalisol B
Catalog No.:BCN9254
CAS No.:169326-06-1
- 2-Methoxy-1,6-dimethyl-5-vinyl-9,10-dihydrophenanthren-7-ol
Catalog No.:BCN9253
CAS No.:2266586-31-4
- Jinflexin A
Catalog No.:BCN9252
CAS No.:2055155-75-2
- 1,6-Dimethyl-5-vinyl-9,10-dihydrophenanthren-2-ol
Catalog No.:BCN9251
CAS No.:745056-83-1
- Dauricumine
Catalog No.:BCN9250
CAS No.:345641-00-1
- Murrayanine
Catalog No.:BCN9249
CAS No.:723-97-7
- Dictysine
Catalog No.:BCN9248
CAS No.:67256-05-7
- Qingyangshengenin 3-O-β-D-cymaropyranosyl-(1→4)-β-D-digitoxopyranoside
Catalog No.:BCN9247
CAS No.:1186628-87-4
- Periplocoside O
Catalog No.:BCN9246
CAS No.:116709-67-2
- Dehydrojuncuenin A
Catalog No.:BCN9245
CAS No.:1161681-26-0
- Lehmbachol D
Catalog No.:BCN9244
CAS No.:913556-40-8
- Cinchonain Ia
Catalog No.:BCN9243
CAS No.:85081-24-9
- 5-O-Methyllatifolin
Catalog No.:BCN9256
CAS No.:18525-14-9
- Matairesinol monoglucoside
Catalog No.:BCN9257
CAS No.:34446-06-5
- threo-Guaiacylglycerol β-coniferyl ether
Catalog No.:BCN9258
CAS No.:168393-18-8
- Jasminoside
Catalog No.:BCN9259
CAS No.:82451-18-1
- Gancaonin O
Catalog No.:BCN9260
CAS No.:129145-53-5
- 9-Oxooctadeca-10,12-dienoic acid
Catalog No.:BCN9261
CAS No.:54232-58-5
- (+)-Dalbergiphenol
Catalog No.:BCN9262
CAS No.:82358-44-9
- Juncuenin B
Catalog No.:BCN9263
CAS No.:1161681-20-4
- Juncuenin A
Catalog No.:BCN9264
CAS No.:1161681-18-0
- Sepiumol E
Catalog No.:BCN9265
CAS No.:2412027-09-7
- 4-Demethyltraxillaside
Catalog No.:BCN9266
CAS No.:1691201-82-7
- Tatsinine
Catalog No.:BCN9267
CAS No.:90038-21-4
Antimycobacterial and Nitric Oxide Production Inhibitory Activities of Triterpenes and Alkaloids from Psychotria nuda (Cham. & Schltdl.) Wawra.[Pubmed:30875889]
Molecules. 2019 Mar 15;24(6). pii: molecules24061026.
A phytochemical study of leaves and twigs of Psychotria nuda resulted in 19 compounds, including five indole alkaloids, N,N,N-trimethyltryptamine, lyaloside, strictosamide, strictosidine, and 5alpha-carboxystrictosidine; two flavonolignans, cinchonain Ia and Cinchonain Ib; an iridoid, roseoside; a sugar, lawsofructose; a coumarin, scopoletin; a diterpene, phytol; three triterpenes, pomolic acid, spinosic acid, and rotungenic acid; and five steroids, sitosterol, stigmasterol, campesterol, beta-sitosterol-3-O-beta-d-glucoside, and beta-stigmasterol-3-O-beta-d-glucoside. Some compounds were evaluated for their in vitro activity against Mycobacterium tuberculosis and their ability to inhibit NO production by macrophages stimulated by lipopolysaccharide (LPS). The compounds pomolic acid, spinosic acid, strictosidine, and 5alpha-carboxystrictosidine displayed antimycobacterial activity with minimum inhibitory concentrations ranging from 7.1 to 19.2 microg/mL. These compounds showed promising inhibitory activity against NO production (IC50 3.22 to 25.5 mug/mL). 5alpha-carboxystrictosidine did not show cytotoxicity against macrophages RAW264.7 up to a concentration of 100 microg/mL. With the exception of strictosamide, this is the first report of the occurrence of these substances in P. nuda.
Polycyclic polyprenylated acylphloroglucinol and phenolic metabolites from the aerial parts of Hypericum elatoides and their neuroprotective and anti-neuroinflammatory activities.[Pubmed:30594026]
Phytochemistry. 2019 Mar;159:65-74.
A phytochemical study on the aerial parts of Hypericum elatoides led to the isolation of a previously undescribed polycyclic polyprenylated acylphloroglucinol derivative, hyperelatone A, seven previously undescribed phenolic metabolites, hyperelatones B-H, along with ten known analogues. The structures of hyperelatones A-H were elucidated by 1D and 2D NMR spectroscopy, HRESIMS experiment, single-crystal X-ray diffraction and comparison of experimental and calculated ECD spectra, as well as chemical derivatization. All compounds were evaluated for their neuroprotective activity against hydrogen peroxide (H2O2)-induced cell injury in rat pheochromocytoma PC-12cells and inhibitory effects on lipopolysaccharide (LPS)-induced nitric oxide (NO) production in BV-2 microglial cells. Hyperelatones B-D and H, Cinchonain Ib, and tenuiside A showed noticeable neuroprotection at concentrations of 1.0-100.0muM. Hyperelatones D, G, and H, (-)-epicatechin, tenuiside A, and (Z)-3-hexenyl-beta-D-glucopyranoside exhibited significant anti-neuroinflammatory activity with IC50 values ranging from 0.75+/-0.02 to 5.83+/-0.23muM.
[Chemical Composition of n-Butanol Fraction from Polygonum amplexicaule var. sinense].[Pubmed:26930981]
Zhong Yao Cai. 2015 Sep;38(9):1872-4.
OBJECTIVE: To study chemical composition of n-butanol fraction from Polygonoum amplexicaule var. sinense. METHODS: TLC,normal-phase silica gel, reveres-phase silica gel, Sephadex-LH and semi-preparative HPLC were used to isolate chemical compositions of n-butanol fraction from Polygonoum amplexicaule var. sinense. RESULTS: Nine compounds were identified as: caffeic acid n-butly ester (1), p-methoxy benzoic acid propyl ester (2),p-E-coumarin quinic acid methyl ester (3),p-Z-coumarin quinic acid methyl ester (4), ethyl ferulate (5), cinchonain I a (6), Cinchonain Ib (7), methyl chlorogenate(8), and 6-O-beta-D-caffeoylglucose (9). CONCLUSION: All compounds are isolated from this genus for the first time.
An overview on antidiabetic medicinal plants having insulin mimetic property.[Pubmed:23569923]
Asian Pac J Trop Biomed. 2012 Apr;2(4):320-30.
Diabetes mellitus is one of the common metabolic disorders acquiring around 2.8% of the world's population and is anticipated to cross 5.4% by the year 2025. Since long back herbal medicines have been the highly esteemed source of medicine therefore, they have become a growing part of modern, high-tech medicine. In view of the above aspects the present review provides profiles of plants (65 species) with hypoglycaemic properties, available through literature source from various database with proper categorization according to the parts used, mode of reduction in blood glucose (insulinomimetic or insulin secretagogues activity) and active phytoconstituents having insulin mimetics activity. From the review it was suggested that, plant showing hypoglycemic potential mainly belongs to the family Leguminoseae, Lamiaceae, Liliaceae, Cucurbitaceae, Asteraceae, Moraceae, Rosaceae and Araliaceae. The most active plants are Allium sativum, Gymnema sylvestre, Citrullus colocynthis, Trigonella foenum greacum, Momordica charantia and Ficus bengalensis. The review describes some new bioactive drugs and isolated compounds from plants such as roseoside, epigallocatechin gallate, beta-pyrazol-1-ylalanine, Cinchonain Ib, leucocyandin 3-O-beta-d-galactosyl cellobioside, leucopelargonidin-3- O-alpha-L rhamnoside, glycyrrhetinic acid, dehydrotrametenolic acid, strictinin, isostrictinin, pedunculagin, epicatechin and christinin-A showing significant insulinomimetic and antidiabetic activity with more efficacy than conventional hypoglycaemic agents. Thus, from the review majorly, the antidiabetic activity of medicinal plants is attributed to the presence of polyphenols, flavonoids, terpenoids, coumarins and other constituents which show reduction in blood glucose levels. The review also discusses the management aspect of diabetes mellitus using these plants and their active principles.
Triterpenoids and flavonoids from Cecropia schreberiana Miq. (Urticaceae).[Pubmed:23459662]
Biochem Syst Ecol. 2013 Jun 1;48:96-99.
Phytochemical investigation of the leaves of Cecropia schreberiana Miq. (Urticaceae) led to the isolation of four triterpenoids (1-4), three flavone C-glycosides (5-7), two flavan-3-ols (8, 9), two flavanolignans (10, 11), and two proanthocyanidins (12, 13). All compounds were isolated from C. schreberiana for the first time. This is the first report demonstrating the presence of arjunolic acid (4), cinchonain Ia (10), and Cinchonain Ib (11) in the Urticaceae family. The occurrence of flavanolignans within the family Urticaceae supports the likelihood that such compounds are more common within the class Magnoliopsida than previously thought.
Cinchonain Ib isolated from Eriobotrya japonica induces insulin secretion in vitro and in vivo.[Pubmed:19397981]
J Ethnopharmacol. 2009 Jul 15;124(2):224-7.
AIMS OF THE STUDY: Eriobotrya japonica leaves had been used traditionally for the treatment of diabetes mellitus by immersing the dried leaves in a hot water drink. Few studies have shown the hypoglycemic effect of Eriobotrya japonica using crude alcoholic extract and isolated methanolic compounds. These studies proposed that the mechanism of action could be by stimulating the beta-islets of Langerhans to secrete insulin, however with no scientific evidence. METHODS: Eriobotrya japonica water extract (EJWE) and the compounds derived from it: Cinchonain Ib, procyanidin B-2, chlorogenic acid and epicatechin, were tested for their effects on insulin secretion from INS-1 cells and following oral administration in rats. RESULTS: The present study showed that EJWE increased significantly (p<0.05) insulin secretion from INS-1 cells in dose-dependent manner. Oral administration of EJWE at 230 mg/kg to rats, however, decreased plasma insulin level for as long as 240 min post-administration and caused a transient drop of blood glucose at 15 and 30 min post-administration. On the other hand, Cinchonain Ib enhanced significantly (p<0.05) insulin secretion from INS-1 cells, whereas epicatechin inhibited significantly (p<0.05) insulin secretion from INS-1 cells. In addition, Cinchonain Ib enhanced significantly (150%: p<0.05) plasma insulin level in rats for as long as 240 min after 108 mg/kg oral administration but did not induce any change in blood glucose level. CONCLUSION: These data indicate that Cinchonain Ib has an insulinotropic effect and suggest the possible use of Cinchonain Ib for managing type 2 diabetes.
Antioxidant phenylpropanoid-substituted epicatechins from Trichilia catigua.[Pubmed:18020420]
J Nat Prod. 2007 Dec;70(12):2010-3.
Two new phenylpropanoid-substituted epicatechins, namely, catiguanin A ( 1) and catiguanin B ( 2), were isolated from the bark of Trichilia catigua along with four known compounds, cinchonain Ia ( 3), Cinchonain Ib ( 4), cinchonain Ic ( 5), and cinchonain Id ( 6). The structures of 1 and 2 were elucidated by analysis of spectroscopic data and by comparison of their NMR data with those of previously reported cinchonains. The isolated compounds exhibited potent antioxidant activity in the alpha,alpha-diphenyl-beta-picrylhydrazyl (DPPH) radical scavenging test, with IC 50 values in the 2.3-9.4 microM range.
Flavanol derivatives from Rhizophora stylosa and their DPPH radical scavenging activity.[Pubmed:17873850]
Molecules. 2007 May 26;12(5):1163-9.
A new acetylated flavanol, 3,7-O-diacetyl (-)-epicatechin (3), and seven known flavanol derivatives, (-)-epicatechin (1), 3-O-acetyl (-)-epicatechin (2), 3,3',4',5,7-O-pentaacetyl (-)-epicatechin (4), (+)-afzelechin (5), (+)-catechin (6), Cinchonain Ib (7), and proanthocyanidin B2 (8), were isolated from the stems and twigs of the mangrove plant Rhizophora stylosa and identified. The crude extract, the different fractions and all of the purified compounds were evaluated for DPPH radical scavenging activity.
A validated higher-performance liquid chromatography method for quantification of cinchonain Ib in bark and phytopharmaceuticals of Trichilia catigua used as Catuaba.[Pubmed:16360665]
J Chromatogr A. 2006 Jun 30;1119(1-2):257-63.
The hydroalcoholic extract, prepared from authentic chopped barks of Trichilia catigua, was evaluated by high-performance liquid chromatography using a diode array detector (200-400 mn). The crude extract was purified by rotation locular counter-current chromatography and the chloroform fraction obtained was clean-up by solid-phase extraction. With the aim of getting preliminary structure information on-line, the methanol fraction thus obtained was analyzed by gradient elution using the diode array detector coupled to a mass spectrometer. The presence of flavalignan in this extract was inferred by the chromatographic band, in the total ion current trace, that had an [M-H](-) = 451. With this information, Cinchonain Ib was isolated as a pure compound from the crude hydroalcoholic extract using a solid-phase extraction procedure for the sample clean-up followed by a semi-preparative separation using the reverse mode of elution. The isolated compound, after complete characterization, was used as an external standard for the development and validation of a method for the analysis of this compound in herbal medicines using the ultraviolet as the detector. The validated method has been successfully applied for quantification of Cinchonain Ib in commercialized herbal medicines sold as Catuaba in Brazil and also in standard chopped barks of T. catigua.
Puerins A and B, two new 8-C substituted flavan-3-ols from Pu-er tea.[Pubmed:16248561]
J Agric Food Chem. 2005 Nov 2;53(22):8614-7.
Pu-er tea is a special treated fermented tea produced from crude green tea, which is prepared from the leaves of Camellia sinensis var. assamica. It is a traditional beverage having been used in China, particularly the southern areas, for a long time. Chemical investigation led to the identification of two new 8-C substituted flavan-3-ols, puerins A (1) and B (2), and two known cinchonain-type phenols, epicatechin-[7,8-bc]-4alpha-(4-hydroxyphenyl)-dihydro-2(3H)-pyranone (3) and Cinchonain Ib (4), together with other seven known phenolic compounds, 2,2',6,6'-tetrahydroxydiphenyl (5), (-)-epicatechin-8-C-beta-d-glucopyranoside (6), (-)-epicatechin (EC) (7), (-)-epigallocatechin (EGC) (8), (+/-)-gallocatechin (GC) (9), gallic acid (10), and myricetin (11), in addition to caffeine (12). Their structures were determined by spectroscopic methods. The compounds 1-5, which might be formed in the postfermentative process of Pu-er tea, were isolated from tea and theaceous plants for the first time.


