J 104129 fumaratePotent, selective M3 antagonist CAS# 257603-40-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 257603-40-0 | SDF | Download SDF |
| PubChem ID | 56972189 | Appearance | Powder |
| Formula | C28H40N2O6 | M.Wt | 500.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 50 mM in ethanol | ||
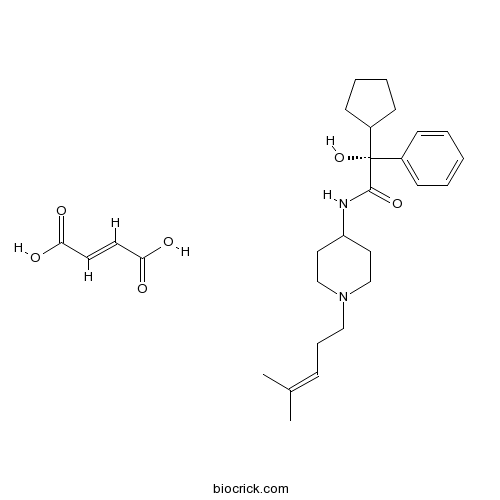
| Chemical Name | (E)-but-2-enedioic acid;(2R)-2-cyclopentyl-2-hydroxy-N-[1-(4-methylpent-3-enyl)piperidin-4-yl]-2-phenylacetamide | ||
| SMILES | CC(=CCCN1CCC(CC1)NC(=O)C(C2CCCC2)(C3=CC=CC=C3)O)C.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | HUCQUCITPHOUAC-DSSYAJFBSA-N | ||
| Standard InChI | InChI=1S/C24H36N2O2.C4H4O4/c1-19(2)9-8-16-26-17-14-22(15-18-26)25-23(27)24(28,21-12-6-7-13-21)20-10-4-3-5-11-20;5-3(6)1-2-4(7)8/h3-5,9-11,21-22,28H,6-8,12-18H2,1-2H3,(H,25,27);1-2H,(H,5,6)(H,7,8)/b;2-1+/t24-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent M3 muscarinic receptor antagonist that displays ~ 120-fold selectivity over M2 receptors (Ki values are 4.2, 19 and 490 nM for human M3, M1 and M2 receptors respectively). Exhibits > 250-fold bronchial selectivity; inhibits ACh-induced bronchoconstriction but not ACh-induced bradycardia (KB values are 3.3 and 170 nM for rat trachea M3 and rat right atria M2 receptors respectively). |

J 104129 fumarate Dilution Calculator

J 104129 fumarate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9975 mL | 9.9874 mL | 19.9748 mL | 39.9497 mL | 49.9371 mL |
| 5 mM | 0.3995 mL | 1.9975 mL | 3.995 mL | 7.9899 mL | 9.9874 mL |
| 10 mM | 0.1997 mL | 0.9987 mL | 1.9975 mL | 3.995 mL | 4.9937 mL |
| 50 mM | 0.0399 mL | 0.1997 mL | 0.3995 mL | 0.799 mL | 0.9987 mL |
| 100 mM | 0.02 mL | 0.0999 mL | 0.1997 mL | 0.3995 mL | 0.4994 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Velutin
Catalog No.:BCN5130
CAS No.:25739-41-7
- KF 38789
Catalog No.:BCC5938
CAS No.:257292-29-8
- Homoarbutin
Catalog No.:BCN2680
CAS No.:25712-94-1
- Tutin
Catalog No.:BCN5129
CAS No.:2571-22-4
- Lonicerin
Catalog No.:BCN8266
CAS No.:25694-72-8
- HU 308
Catalog No.:BCC5971
CAS No.:256934-39-1
- Cimiracemoside C
Catalog No.:BCN5128
CAS No.:256925-92-5
- CCK Octapeptide, non-sulfated
Catalog No.:BCC5709
CAS No.:25679-24-7
- 3,2'-Dihydroxy-4,4'-dimethoxychalcone
Catalog No.:BCN7742
CAS No.:2567-65-9
- Cannabigerol
Catalog No.:BCN5127
CAS No.:25654-31-3
- Aponorhyoscine
Catalog No.:BCN1871
CAS No.:25650-56-0
- Nantenine
Catalog No.:BCN7788
CAS No.:2565-01-7
- CP 154526
Catalog No.:BCC7481
CAS No.:257639-98-8
- H-D-Glu(OBzl)-OH
Catalog No.:BCC2939
CAS No.:2578-33-8
- Pelitinib (EKB-569)
Catalog No.:BCC1118
CAS No.:257933-82-7
- Gemfibrozil
Catalog No.:BCC4783
CAS No.:25812-30-0
- LEP (116-130) (mouse)
Catalog No.:BCC1016
CAS No.:258276-95-8
- Ghrelin (human)
Catalog No.:BCC7076
CAS No.:258279-04-8
- IEM 1925 dihydrobromide
Catalog No.:BCC7885
CAS No.:258282-23-4
- Ghrelin (rat)
Catalog No.:BCC5767
CAS No.:258338-12-4
- H-D-Cys(Trt)-OH
Catalog No.:BCC2914
CAS No.:25840-82-8
- Liensinine
Catalog No.:BCN6337
CAS No.:2586-96-1
- Hoechst 33258 analog
Catalog No.:BCC1624
CAS No.:258843-62-8
- Yunaconitoline
Catalog No.:BCN6703
CAS No.:259099-25-7
Muscarinic cholinoreceptors (M1-, M2-, M3- and M4-type) modulate the acetylcholine secretion in the frog neuromuscular junction.[Pubmed:28408330]
Neurosci Lett. 2017 May 10;649:62-69.
Muscarinic cholinoreceptors regulate the neurosecretion process in vertebrate neuromuscular junctions. The diversity of muscarinic effects on acetylcholine (ACh) secretion may be attributed to the different muscarinic subtypes involved in this process. In the present study, the location of five muscarinic receptor subtypes (M1, M2, M3, M4 and M5) on the motor nerve terminals of frog cutaneous pectoris muscle was shown using specific polyclonal antibodies. The modulatory roles of these receptors were investigated via assessment of the effects of muscarine and specific muscarinic antagonists on the quantal content of endplate currents (EPCs) and the time course of secretion, which was estimated from the distribution of "real" synaptic delays of EPCs recorded in a low Ca(2+)/high Mg(2+) solution. The agonist muscarine decreased the EPC quantal content and synchronized the release process. The depressing action of muscarine on the EPC quantal content was abolished only by pretreatment of the preparation with the M3 blockers 4-DAMP (1,1-Dimethyl-4-diphenylacetoxypiperidinium iodide) and J 104129 fumarate ((alphaR)-alpha-Cyclopentyl-alpha-hydroxy-N-[1-(4-methyl-3-pentenyl)-4-piperidiny l]benzeneacetamide fumarate). Moreover, antagonists of the M1, M2, M3 and M4 receptors per se diminished the intensity of secretion, which suggests a putative up-regulation of the release by endogenous ACh.
Inflammation triggers constitutive activity and agonist-induced negative responses at M(3) muscarinic receptor in dental pulp.[Pubmed:21238800]
J Endod. 2011 Feb;37(2):185-90.
The purpose of this study was to investigate whether the inflammation of rat dental pulp induces the muscarinic acetylcholine receptor (mAChR) constitutive receptor activity. Pulpitis was induced with bacterial lipolysaccharide in rat incisors dental pulp. Saturation assay with [(3)H]-quinuclidinyl benzilate ([(3)H] QNB), competitive binding with different mAChR antagonist subtypes, and nitric oxide synthase (NOS) activity were performed. A drastic change in expression and response to mAChR subtypes was observed in pulpitis. Inflamed pulp expressed high number of M(3) mAChR of high affinity, whereas the M(1) mAChR is the main subtype displayed in normal pulp. Consistent with the identification of the affinity constant (Ki) of M(3) and Ki of M(1) in both pulpitis and in normal pulps are the differences in the subtype functionality of these cells. In pulpitis, pilocarpine (1 x 10(-11) mol/L to 5 x 10(-9) mol/L) exerted an inhibitory action on NOS activity that was blocked by J 104129 fumarate (highest selective affinity to M(3) mAChR). In normal pulps, pilocarpine (1 x 10(-11) mol/L to 5 x 10(-9) mol/L) has no effect. NOS basal activity was 5.9 times as high in pulpitis as in the normal pulp as a result of the activation of inducible NOS. The irreversible pulpitis could induce a mAChR alteration, increasing the high-affinity receptor density and transduction-coupling efficiency of inducible NOS activity, leading to a spontaneously active conformation of the receptor. Pilocarpine acting as an inverse agonist might be useful therapeutically to prevent necrosis and subsequent loss of dental pulp.
Discovery of a muscarinic M3 receptor antagonist with high selectivity for M3 over M2 receptors among 2-[(1S,3S)-3-sulfonylaminocyclopentyl]phenylacetamide derivatives.[Pubmed:10819171]
Bioorg Med Chem. 2000 Apr;8(4):825-32.
In the course of developing a metabolically stable M3 receptor antagonist from the prototype antagonist, J-104129 (1), introduction of certain substituents into the cyclopentane ring of 1 was found to be effective not only in improving metabolic stability but also in greatly enhancing the subtype selectivity. Among the cyclopentane analogues, sulfonamide derivatives (10f) and (10g) displayed 160- and 310-fold selectivity for M3 over M2 receptors, and both were significantly more selective than the prototype antagonist (120-fold). Subsequent derivatization of the sulfonamide series led to the highly selective M3 receptor antagonists (10h, 10i and 10j) with >490-fold selectivity for M3 over M2 receptors. Among them, p-nitrophenylsulfonamide (J-107320, 10h) exhibited 1100-fold selectivity for M3 receptors (Ki = 2.5 nM) over M2 receptors (Ki = 2800 nM) in the human muscarinic receptor binding assay using [3H]-NMS as a radio ligand.
J-104129, a novel muscarinic M3 receptor antagonist with high selectivity for M3 over M2 receptors.[Pubmed:10632066]
Bioorg Med Chem. 1999 Nov;7(11):2555-67.
A new class of 4-acetamidopiperidine derivatives has been synthesized and investigated for human muscarinic receptor subtype selectivity. Introduction of a hydrocarbon chain of appropriate length into the piperidine nitrogen of the racemic N-(piperidin-4-yl)-2-cyclobutyl-2-hydroxy-2-phenylacetamide platform conferred up to 70-fold selectivity for human muscarinic M3 receptors over M2 receptors. Subsequent synthetic derivatizations resulted in highly potent M3 receptor antagonists with selectivity greater than two orders of magnitude for M3 over M2 receptors, from which the analogue 4r was selected. Preparation of both enantiomers of 4r led to the identification of (2R)-N-[1-(4-methyl-3-pentenyl)piperidin-4-yl]-2-cyclopentyl-2-hyd roxy-2-phenylacetamide (J-104129, (R)-4r), which exhibited 120-fold selectivity for M3 receptors (Ki = 4.2 nM) over M2 receptors (Ki = 490 nM). In isolated rat trachea, (R)-4r potently and specifically antagonized acetylcholine (ACh)-induced responses with a K(B) value of 3.3 nM. The highly subtype-selective profile was also seen in isolated rat tissue assays (50-fold) and in anesthetized rats (> 250-fold). Oral administration of J-104129 ((R)-4r) antagonized ACh-induced bronchoconstriction with an ED50 value of 0.58 mg/kg in rats. Thus, J-104129 ((R)-4r) may effectively facilitate bronchodilation in the treatment of obstructive airway disease.


