Isoliquiritin apiosideCAS# 120926-46-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 120926-46-7 | SDF | Download SDF |
| PubChem ID | 6442433 | Appearance | Powder |
| Formula | C26H30O13 | M.Wt | 550.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
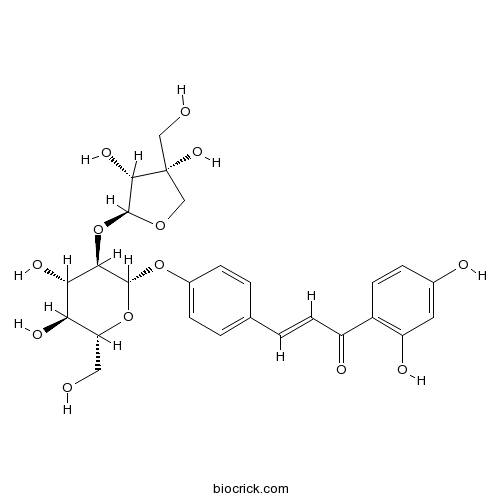
| Chemical Name | (E)-3-[4-[(2S,3R,4S,5S,6R)-3-[(2S,3R,4R)-3,4-dihydroxy-4-(hydroxymethyl)oxolan-2-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-1-(2,4-dihydroxyphenyl)prop-2-en-1-one | ||
| SMILES | C1C(C(C(O1)OC2C(C(C(OC2OC3=CC=C(C=C3)C=CC(=O)C4=C(C=C(C=C4)O)O)CO)O)O)O)(CO)O | ||
| Standard InChIKey | VMMVZVPAYFZNBM-KVFWHIKKSA-N | ||
| Standard InChI | InChI=1S/C26H30O13/c27-10-19-20(32)21(33)22(39-25-23(34)26(35,11-28)12-36-25)24(38-19)37-15-5-1-13(2-6-15)3-8-17(30)16-7-4-14(29)9-18(16)31/h1-9,19-25,27-29,31-35H,10-12H2/b8-3+/t19-,20-,21+,22-,23+,24-,25+,26-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isoliquiritin apioside with marked potential to combat oxidative stress-induced genotoxicity. |
| Targets | ROS | 5-HT Receptor |
| In vitro | Evaluation of antigenotoxic activity of isoliquiritin apioside from Glycyrrhiza glabra L.[Pubmed: 19490840]Toxicol In Vitro. 2009 Jun;23(4):680-6.Prevention of manifestation of events characteristic of carcinogenesis is being emphasized a rational strategy to combat cancer. Reactive oxygen species (ROS) play an important role in tumor initiation through oxidative damage of DNA. |
| Kinase Assay | Antitussive principles of Glycyrrhizae radix, a main component of the Kampo preparations Bakumondo-to (Mai-men-dong-tang).[Pubmed: 12782198]Eur J Pharmacol. 2003 May 23;469(1-3):159-63.We attempted to elucidate the antitussive principles of Glycyrrhizae radix, a main component of Bakumondo-to (Mai-men-dong-tang). Although the 50% methanol-eluted fraction (100 mg/kg, p.o.) caused a more than 60% reduction in the number of capsaicin-induced coughs, neither the water-eluted nor 100% ethanol-eluted fractions of water extract of G. radix had antitussive effects. The water extract of G. radix contained high levels of liquiritin, liquiritin apioside, isoliquiritin, Isoliquiritin apioside and glycyrrhizin. On the other hand, the 50% methanol-eluted fraction contained mainly liquiritin and liquiritin apioside, but not the other compounds. Liquiritin apioside (3-30 mg/kg, p.o.), but not liquiritin, isoliquiritin, Isoliquiritin apioside or glycyrrhizin, dose-dependently inhibited the number of coughs. Methysergide, a serotonin receptor antagonist, antagonized the antitussive effect of liquiritin apioside. However, the antitussive effect of liquiritin apioside was not antagonized by naloxone. Pretreatment with glibenclamide (3 mg/kg, i.p.), an ATP-sensitive potassium channel blocker, also significantly reduced the antinociceptive effect of liquiritin apioside. These results suggest that G. radix contains a potent antitussive compound, liquilitin apioside, whose antitussive effect may depend on both peripheral and central mechanisms. |

Isoliquiritin apioside Dilution Calculator

Isoliquiritin apioside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8165 mL | 9.0827 mL | 18.1653 mL | 36.3306 mL | 45.4133 mL |
| 5 mM | 0.3633 mL | 1.8165 mL | 3.6331 mL | 7.2661 mL | 9.0827 mL |
| 10 mM | 0.1817 mL | 0.9083 mL | 1.8165 mL | 3.6331 mL | 4.5413 mL |
| 50 mM | 0.0363 mL | 0.1817 mL | 0.3633 mL | 0.7266 mL | 0.9083 mL |
| 100 mM | 0.0182 mL | 0.0908 mL | 0.1817 mL | 0.3633 mL | 0.4541 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PF-03394197(Oclacitinib)
Catalog No.:BCC6474
CAS No.:1208319-26-9
- N6022
Catalog No.:BCC4127
CAS No.:1208315-24-5
- Ketone Ester
Catalog No.:BCC1677
CAS No.:1208313-97-6
- VU 0365114
Catalog No.:BCC6164
CAS No.:1208222-39-2
- CaMKII-IN-1
Catalog No.:BCC5530
CAS No.:1208123-85-6
- Quassidine B
Catalog No.:BCN7022
CAS No.:1207862-37-0
- Gynosaponin I
Catalog No.:BCN4078
CAS No.:1207861-69-5
- Huperzine A
Catalog No.:BCN1058
CAS No.:120786-18-7
- 3,2'-Epilarixinol
Catalog No.:BCN6496
CAS No.:1207671-28-0
- LDV FITC
Catalog No.:BCC6229
CAS No.:1207610-07-8
- 5-OMe-UDP trisodium salt
Catalog No.:BCC6153
CAS No.:1207530-98-0
- BMN 673
Catalog No.:BCC2205
CAS No.:1207456-01-6
- FPL 64176
Catalog No.:BCC7050
CAS No.:120934-96-5
- 3-Deazaneplanocin A (DZNep) hydrochloride
Catalog No.:BCC3604
CAS No.:120964-45-6
- Vanillin
Catalog No.:BCN2605
CAS No.:121-33-5
- Vanillic acid
Catalog No.:BCN6105
CAS No.:121-34-6
- (-)-Terreic acid
Catalog No.:BCC7051
CAS No.:121-40-4
- Benzethonium Chloride
Catalog No.:BCC4635
CAS No.:121-54-0
- N-Acetylsulfanilyl chloride
Catalog No.:BCC9084
CAS No.:121-60-8
- 2-Amino-5-nitrothiazole
Catalog No.:BCC8538
CAS No.:121-66-4
- Propyl gallate
Catalog No.:BCN8431
CAS No.:121-79-9
- 3'-Nitroacetophenone
Catalog No.:BCN2256
CAS No.:121-89-1
- ST 1936 oxalate
Catalog No.:BCC7919
CAS No.:1210-81-7
- N-Acetyl-5-Hydroxytryptamine
Catalog No.:BCC9080
CAS No.:1210-83-9
Antitussive principles of Glycyrrhizae radix, a main component of the Kampo preparations Bakumondo-to (Mai-men-dong-tang).[Pubmed:12782198]
Eur J Pharmacol. 2003 May 23;469(1-3):159-63.
We attempted to elucidate the antitussive principles of Glycyrrhizae radix, a main component of Bakumondo-to (Mai-men-dong-tang). Although the 50% methanol-eluted fraction (100 mg/kg, p.o.) caused a more than 60% reduction in the number of capsaicin-induced coughs, neither the water-eluted nor 100% ethanol-eluted fractions of water extract of G. radix had antitussive effects. The water extract of G. radix contained high levels of liquiritin, liquiritin apioside, isoliquiritin, Isoliquiritin apioside and glycyrrhizin. On the other hand, the 50% methanol-eluted fraction contained mainly liquiritin and liquiritin apioside, but not the other compounds. Liquiritin apioside (3-30 mg/kg, p.o.), but not liquiritin, isoliquiritin, Isoliquiritin apioside or glycyrrhizin, dose-dependently inhibited the number of coughs. Methysergide, a serotonin receptor antagonist, antagonized the antitussive effect of liquiritin apioside. However, the antitussive effect of liquiritin apioside was not antagonized by naloxone. Pretreatment with glibenclamide (3 mg/kg, i.p.), an ATP-sensitive potassium channel blocker, also significantly reduced the antinociceptive effect of liquiritin apioside. These results suggest that G. radix contains a potent antitussive compound, liquilitin apioside, whose antitussive effect may depend on both peripheral and central mechanisms.
Evaluation of antigenotoxic activity of isoliquiritin apioside from Glycyrrhiza glabra L.[Pubmed:19490840]
Toxicol In Vitro. 2009 Jun;23(4):680-6.
Prevention of manifestation of events characteristic of carcinogenesis is being emphasized a rational strategy to combat cancer. Reactive oxygen species (ROS) play an important role in tumor initiation through oxidative damage of DNA. In search for lead molecules in cancer chemoprevention from natural products, a fraction 'Rlicca' isolated from Glycyrrhiza glabra was studied for modulatory effect against hydrogen peroxide and 4-nitroquinoline-N-oxide induced genotoxicity in Escherichiacoli PQ37 using SOS chromotest and in human peripheral blood lymphocytes using the Comet assay. The fraction 'Rlicca' at a concentration of 191 microM decreased the SOS inducing potency (SOSIP) of hydrogen peroxide (1.0mM) and NQO (20 microg/ml) by 83.72% and 68.77%, respectively. In the human blood lymphocytes, 'Rlicca' reduced the tail moment induced by hydrogen peroxide (25 microM) and NQO (5 microg/ml) by 88.04% and 76.64%, respectively, using the Comet assay. The spectroscopic data of 'Rlicca' fraction revealed it to be Isoliquiritin apioside, a chalcone oligoglycoside. This is the first report of Isoliquiritin apioside with marked potential to combat oxidative stress-induced genotoxicity.


