IrenoloneCAS# 149184-19-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 149184-19-0 | SDF | Download SDF |
| PubChem ID | 2754650 | Appearance | Powder |
| Formula | C19H12O3 | M.Wt | 288.30 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
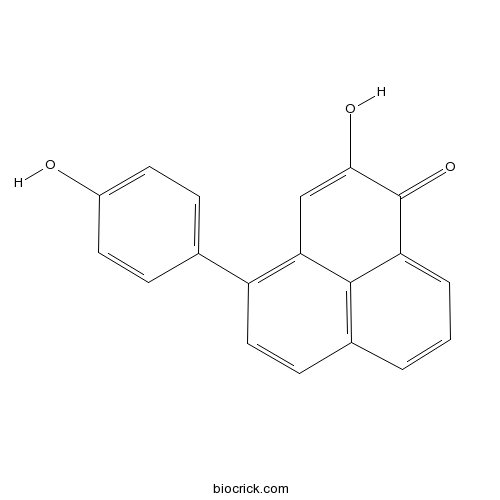
| Chemical Name | 2-hydroxy-4-(4-hydroxyphenyl)phenalen-1-one | ||
| SMILES | C1=CC2=C3C(=C1)C(=O)C(=CC3=C(C=C2)C4=CC=C(C=C4)O)O | ||
| Standard InChIKey | UQMKPTIDKHEGFW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H12O3/c20-13-7-4-11(5-8-13)14-9-6-12-2-1-3-15-18(12)16(14)10-17(21)19(15)22/h1-10,20-21H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Irenolone is a natural product from Musa itinerans Cheesm. |
| In vitro | Diarylheptanoids and phenylphenalenones from Musa itinerans fruits.[Pubmed: 24766994]Phytochemistry. 2014 Jul;103:171-177.
Changes in the content and biosynthesis of phytoalexins in banana fruit.[Pubmed: 11129580 ]Biosci Biotechnol Biochem. 2000 Oct;64(10):2089-98.
|

Irenolone Dilution Calculator

Irenolone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4686 mL | 17.343 mL | 34.6861 mL | 69.3722 mL | 86.7152 mL |
| 5 mM | 0.6937 mL | 3.4686 mL | 6.9372 mL | 13.8744 mL | 17.343 mL |
| 10 mM | 0.3469 mL | 1.7343 mL | 3.4686 mL | 6.9372 mL | 8.6715 mL |
| 50 mM | 0.0694 mL | 0.3469 mL | 0.6937 mL | 1.3874 mL | 1.7343 mL |
| 100 mM | 0.0347 mL | 0.1734 mL | 0.3469 mL | 0.6937 mL | 0.8672 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-(2,4-Dihydroxybenzyl)-5-hydroxy-7,8-dimethoxy-6-methylchroman-4-one
Catalog No.:BCN6634
CAS No.:149180-48-3
- Eriodictyol chalcone
Catalog No.:BCN8276
CAS No.:14917-41-0
- Homaloside D
Catalog No.:BCN1661
CAS No.:149155-19-1
- Cratoxylone
Catalog No.:BCN3875
CAS No.:149155-01-1
- Brugine
Catalog No.:BCN1899
CAS No.:14912-30-2
- AG 825
Catalog No.:BCC7113
CAS No.:149092-50-2
- Brusatol
Catalog No.:BCN8278
CAS No.:14907-98-3
- H-D-Trp-OMe.HCl
Catalog No.:BCC3118
CAS No.:14907-27-8
- (S)-WAY 100135 dihydrochloride
Catalog No.:BCC6993
CAS No.:149007-54-5
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- D-Isomenthol
Catalog No.:BCN8540
CAS No.:23283-97-8
- Gallic acid
Catalog No.:BCN1668
CAS No.:149-91-7
- CGP 54626 hydrochloride
Catalog No.:BCC6934
CAS No.:149184-21-4
- CGP 55845 hydrochloride
Catalog No.:BCC5737
CAS No.:149184-22-5
- (±)-Decanoylcarnitine chloride
Catalog No.:BCC6659
CAS No.:14919-36-9
- (±)-Myristoylcarnitine chloride
Catalog No.:BCC6698
CAS No.:14919-38-1
- Benserazide HCl
Catalog No.:BCC4468
CAS No.:14919-77-8
- H-Abu-OH
Catalog No.:BCC3198
CAS No.:1492-24-6
- Z-Ser(Tos)-OMe
Catalog No.:BCC2741
CAS No.:1492-52-0
- Arcaine sulfate
Catalog No.:BCC6631
CAS No.:14923-17-2
- Neotripterifordin
Catalog No.:BCN7477
CAS No.:149249-32-1
- De-4'-O-methylyangambin
Catalog No.:BCN1662
CAS No.:149250-48-6
- 1-Dehydroxy-23-deoxojessic acid
Catalog No.:BCN1663
CAS No.:149252-87-9
- pp60 c-src (521-533) (phosphorylated)
Catalog No.:BCC5851
CAS No.:149299-77-4
Diarylheptanoids and phenylphenalenones from Musa itinerans fruits.[Pubmed:24766994]
Phytochemistry. 2014 Jul;103:171-177.
Two diarylheptanoids, musaitinerins A and B, one heterodimeric phenylphenalenone musaitinerone and four known phenylphenalenones, identified as 4-hydroxy-2-methoxy-9-phenyl-1H-phenalen-1-one, musanolone E, hydroxyanigorufone and Irenolone were isolated from the fruits of Musa itinerans Cheesm. Their structures were elucidated using spectroscopic analyses. The antimicrobial activity of these compounds was evaluated against Escherichia coli, Staphylococcus aureus and Candida albicans; the cytotoxic activity of these compounds was also evaluated against human erythromyeloblastoid leukemia (K562) and human alveolar carcinoma epithelial (A549) cell lines, respectively. Musaitinerone and musanolone E exhibited weak effects against the A549 cell line, as compared with adriamycin. However, these two compounds did not exhibit any growth inhibition against K562 cells, S. aureus, E. coli or C. albicans. The other compounds were inactive against all of the tested cell lines and microorganisms, even at concentrations as high as 50 muM.
[Active compounds from rhizomes of Musa basjoo].[Pubmed:21141492]
Zhongguo Zhong Yao Za Zhi. 2010 Sep;35(18):2424-7.
OBJECTIVE: To study the active compounds from the rhizomes of Musa basjoo. METHOD: Antioxidant and alpha-glucosidase inhibitory activity of different extracts were tested. Using bioassay-guided fractionation, the chemical constituents in EtOAC extracts were isolated by column chromatography and identified by MS and NMR spectroscopy. RESULT: Five compounds were isolated and identified as 2',3, 4'-trihydroxyflavone (1), 3,3'-bis-hydroxyanigorufone (2), Irenolone (3), 4-dihydroxy-9-(4'-hydroxyphenyl)-phenalenone (4) and 3,4-dihydroxybenzaldehyde (5). Compound 1(IC50 8.61 mg x L(-1)), 3 (IC50 19.55 mg x L(-1)) and 5 (IC50 1.1 mg x L(-1)) had antioxidant activity. Compound 2 (IC50 24.15 mg x L(-1)) and 4(IC50 2.81 mg x L(-1)) had alpha-glucosidase inhibitory activity. Compound 5 showed MIC of 0.078, 0.313, 0.039 microg/disc against SA, MRSA and ESBLs, respectively. CONCLUSION: Compound 1-5 were isolated from this plant for the first time. Compound 5 was isolated from the genus Musa for the first time. All compound except 5 were first reported about activity.
Changes in the content and biosynthesis of phytoalexins in banana fruit.[Pubmed:11129580]
Biosci Biotechnol Biochem. 2000 Oct;64(10):2089-98.
Changes in the phytoalexin content in unripe fruit of banana, Musa acuminata, were analyzed after various treatments. The results show that level of hydroxyanigorufone started to increase 1-2 day after either wounding or inoculation with conidia of Colletotrichum musae. Inoculation followed by wounding induced the formation of many other phenylphenalenones. The accumulation of hydroxyanigorufone decreased, after its transient maximum, on ripening by exposure of the wounded fruit to ethylene. The level of production of hydroxyanigorufone in ripe fruit treated by wounding and/or by inoculation was much lower than that in unripe fruit. 2-Aminooxyacetic acid, an inhibitor of phenylalanine ammonia-lyase (PAL), inhibited the accumulation of hydroxyanigorufone in wounded fruit, and the PAL activity increased after wounding and ethylene treatment, respectively. Feeding experiments with [1-(13)C] and [2-(13)C]cinnamic acids, and [2-(13)C]malonate show that two molecules of cinnamic acid and one of malonate were incorporated into each molecule of hydroxyanigorufone. The phytoalexins isolated from fruit to which deuterated hydroxyanigorufone and Irenolone had been administered revealed that 2-(4'-hydroxyphenyl)-1,8-naphthalic anhydride was biosynthesized from hydroxyanigorufone rather than from Irenolone.


