CGP 54626 hydrochloridePotent, selective GABAB antagonist CAS# 149184-21-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 149184-21-4 | SDF | Download SDF |
| PubChem ID | 197583 | Appearance | Powder |
| Formula | C18H29Cl3NO3P | M.Wt | 444.76 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO and to 10 mM in ethanol with gentle warming | ||
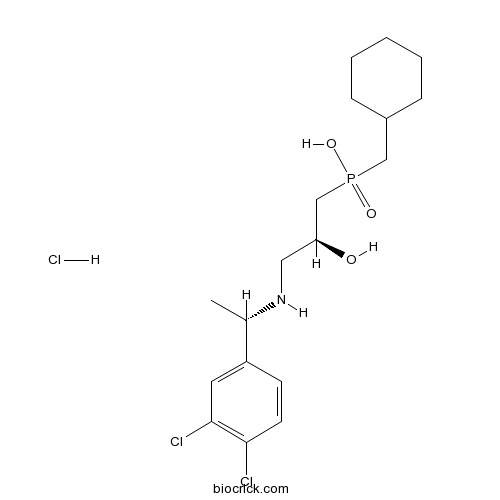
| Chemical Name | cyclohexylmethyl-[(2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino]-2-hydroxypropyl]phosphinic acid;hydrochloride | ||
| SMILES | CC(C1=CC(=C(C=C1)Cl)Cl)NCC(CP(=O)(CC2CCCCC2)O)O.Cl | ||
| Standard InChIKey | ZQCFHOVIXCJPLE-LINSIKMZSA-N | ||
| Standard InChI | InChI=1S/C18H28Cl2NO3P.ClH/c1-13(15-7-8-17(19)18(20)9-15)21-10-16(22)12-25(23,24)11-14-5-3-2-4-6-14;/h7-9,13-14,16,21-22H,2-6,10-12H2,1H3,(H,23,24);1H/t13-,16-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A potent, selective GABAB receptor antagonist (IC50 = 4 nM). |

CGP 54626 hydrochloride Dilution Calculator

CGP 54626 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2484 mL | 11.242 mL | 22.484 mL | 44.9681 mL | 56.2101 mL |
| 5 mM | 0.4497 mL | 2.2484 mL | 4.4968 mL | 8.9936 mL | 11.242 mL |
| 10 mM | 0.2248 mL | 1.1242 mL | 2.2484 mL | 4.4968 mL | 5.621 mL |
| 50 mM | 0.045 mL | 0.2248 mL | 0.4497 mL | 0.8994 mL | 1.1242 mL |
| 100 mM | 0.0225 mL | 0.1124 mL | 0.2248 mL | 0.4497 mL | 0.5621 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Irenolone
Catalog No.:BCN7146
CAS No.:149184-19-0
- 3-(2,4-Dihydroxybenzyl)-5-hydroxy-7,8-dimethoxy-6-methylchroman-4-one
Catalog No.:BCN6634
CAS No.:149180-48-3
- Eriodictyol chalcone
Catalog No.:BCN8276
CAS No.:14917-41-0
- Homaloside D
Catalog No.:BCN1661
CAS No.:149155-19-1
- Cratoxylone
Catalog No.:BCN3875
CAS No.:149155-01-1
- Brugine
Catalog No.:BCN1899
CAS No.:14912-30-2
- AG 825
Catalog No.:BCC7113
CAS No.:149092-50-2
- Brusatol
Catalog No.:BCN8278
CAS No.:14907-98-3
- H-D-Trp-OMe.HCl
Catalog No.:BCC3118
CAS No.:14907-27-8
- (S)-WAY 100135 dihydrochloride
Catalog No.:BCC6993
CAS No.:149007-54-5
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- D-Isomenthol
Catalog No.:BCN8540
CAS No.:23283-97-8
- CGP 55845 hydrochloride
Catalog No.:BCC5737
CAS No.:149184-22-5
- (±)-Decanoylcarnitine chloride
Catalog No.:BCC6659
CAS No.:14919-36-9
- (±)-Myristoylcarnitine chloride
Catalog No.:BCC6698
CAS No.:14919-38-1
- Benserazide HCl
Catalog No.:BCC4468
CAS No.:14919-77-8
- H-Abu-OH
Catalog No.:BCC3198
CAS No.:1492-24-6
- Z-Ser(Tos)-OMe
Catalog No.:BCC2741
CAS No.:1492-52-0
- Arcaine sulfate
Catalog No.:BCC6631
CAS No.:14923-17-2
- Neotripterifordin
Catalog No.:BCN7477
CAS No.:149249-32-1
- De-4'-O-methylyangambin
Catalog No.:BCN1662
CAS No.:149250-48-6
- 1-Dehydroxy-23-deoxojessic acid
Catalog No.:BCN1663
CAS No.:149252-87-9
- pp60 c-src (521-533) (phosphorylated)
Catalog No.:BCC5851
CAS No.:149299-77-4
- Cytochalasin B
Catalog No.:BCN7084
CAS No.:14930-96-2
Antinociception produced by systemic R(+)-baclofen hydrochloride is attenuated by CGP 35348 administered to the spinal cord or ventromedial medulla of rats.[Pubmed:8773775]
Brain Res. 1996 Apr 29;718(1-2):129-37.
This study examined the sites in the central nervous system at which subcutaneously-administered R(+)-baclofen hydrochloride (baclofen), the most active isomer of this prototypic gamma-aminobutyric acid (GABA)B receptor agonist, acts to produce antinociception in the rat. To determine whether baclofen acts in the spinal cord, either saline or the GABAB receptor antagonist CGP 35348 was injected intrathecally in rats pretreated 24 min earlier with 1 or 3 mg/kg s.c. baclofen. Intrathecal (i.t.) injection of 3 or 10 micrograms of CGP 35348 antagonized the increase in tail-flick and hot-plate latency produced by either dose of baclofen. To determine whether baclofen acts at sites in the ventromedial medulla (VMM), either saline or CGP 35348 was microinjected in the nucleus raphe magnus or nucleus reticularis gigantocellularis pars alpha of rats pretreated 24 min earlier with 1 or 3 mg/kg s.c. baclofen. Microinjection of 0.5 or 3 micrograms of CGP 35348 at sites in the VMM produced at best only a very modest attenuation of the antinociceptive effects of baclofen. These data suggest that systemically-administered baclofen acts at sites in both the spinal cord and the VMM, but that its antinociceptive effects are likely to be mediated to a greater extent by a spinal, rather than medullary site of action. However, a definitive comparison of the relative contribution of GABAB receptors in these two regions is precluded by differences in the diffusion and concentrations of the antagonist in the spinal cord and brainstem. Finally, microinjection of 0.5 or 3.0 micrograms of CGP 35348 in the nucleus raphe magnus or nucleus reticularis gigantocellularis pars alpha of saline-pretreated rats did not alter tail-flick or hot-plate latency. This finding suggests that, unlike GABAA receptors, GABAB receptors do not mediate the tonic GABAergic input to neurons in these nuclei.
GABA(B)-receptor subtypes assemble into functional heteromeric complexes.[Pubmed:9872317]
Nature. 1998 Dec 17;396(6712):683-7.
B-type receptors for the neurotransmitter GABA (gamma-aminobutyric acid) inhibit neuronal activity through G-protein-coupled second-messenger systems, which regulate the release of neurotransmitters and the activity of ion channels and adenylyl cyclase. Physiological and biochemical studies show that there are differences in drug efficiencies at different GABA(B) receptors, so it is expected that GABA(B)-receptor (GABA(B)R) subtypes exist. Two GABA(B)-receptor splice variants have been cloned (GABA(B)R1a and GABA(B)R1b), but native GABA(B) receptors and recombinant receptors showed unexplained differences in agonist-binding potencies. Moreover, the activation of presumed effector ion channels in heterologous cells expressing the recombinant receptors proved difficult. Here we describe a new GABA(B) receptor subtype, GABA(B)R2, which does not bind available GABA(B) antagonists with measurable potency. GABA(B)R1a, GABA(B)R1b and GABA(B)R2 alone do not activate Kir3-type potassium channels efficiently, but co-expression of these receptors yields a robust coupling to activation of Kir3 channels. We provide evidence for the assembly of heteromeric GABA(B) receptors in vivo and show that GABA(B)R2 and GABA(B)R1a/b proteins immunoprecipitate and localize together at dendritic spines. The heteromeric receptor complexes exhibit a significant increase in agonist- and partial-agonist-binding potencies as compared with individual receptors and probably represent the predominant native GABA(B) receptor. Heteromeric assembly among G-protein-coupled receptors has not, to our knowledge, been described before.
The action of new potent GABAB receptor antagonists in the hemisected spinal cord preparation of the rat.[Pubmed:8390938]
Eur J Pharmacol. 1993 Apr 22;235(1):153-5.
CGP 52432 (3-N-(3,4-dichlorobenzyl)aminopropyl-P-diethoxymethylphosphinic acid), CGP 54062 (3-N[1-(R,S)-(3,4-dichlorophenyl)ethyl]amino-2-(S)-hydroxypropyl-P-benzy l- phosphinic acid), CGP 54626 (3-N[[1-(S)-(3,4-dichlorophenyl)ethyl]amino-2-(S)- hydroxypropyl-P-cyclohexylmethylphosphinic acid) and CGP 55845 (3-N[1-(S)-(3,4-dichlorophenyl)ethyl]amino-2-(S)- hydroxypropyl-P-benzyl-phosphinic acid) are novel selective GABAB receptor antagonist. The apparent Kd values for the complex formed between the GABAB receptor and these compounds were determined using the monosynaptic reflex in the hemisected rat spinal cord preparation in vitro. CGP 55845 was found to be the most potent GABAB receptor antagonist tested (apparent Kd = 30 nM). On the same preparation 0.3 microM CGP 55845 was equipotent with 100 microM of CGP 35348 (P-(3-aminopropyl)-P-diethoxymethyl-phosphinic acid) for reversal of the depressant action of (R)-(-)-baclofen.


