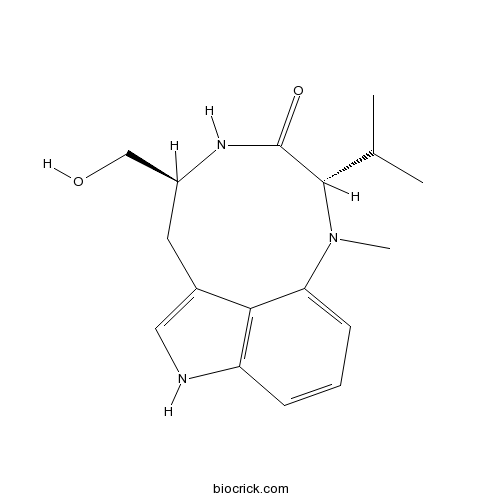
(-)-Indolactam VCAS# 90365-57-4 |
- INCB3344
Catalog No.:BCC1648
CAS No.:1262238-11-8
- RS 504393
Catalog No.:BCC1910
CAS No.:300816-15-3
- MK-0812
Catalog No.:BCC1755
CAS No.:624733-88-6
- INCB 3284 dimesylate
Catalog No.:BCC1646
CAS No.:887401-93-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 90365-57-4 | SDF | Download SDF |
| PubChem ID | 105000 | Appearance | Powder |
| Formula | C17H23N3O2 | M.Wt | 301.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Indolactam V | ||
| Solubility | DMSO : 50 mg/mL (165.90 mM; Need ultrasonic) | ||
| SMILES | CC(C)C1C(=O)NC(CC2=CNC3=C2C(=CC=C3)N1C)CO | ||
| Standard InChIKey | LUZOFMGZMUZSSK-LRDDRELGSA-N | ||
| Standard InChI | InChI=1S/C17H23N3O2/c1-10(2)16-17(22)19-12(9-21)7-11-8-18-13-5-4-6-14(15(11)13)20(16)3/h4-6,8,10,12,16,18,21H,7,9H2,1-3H3,(H,19,22)/t12-,16-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Protein kinase C activator. Exhibits tumor promoting activity. Directs differentiation of human embryonic stem cells (ESCs) into pancreatic progenitors. |

(-)-Indolactam V Dilution Calculator

(-)-Indolactam V Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3181 mL | 16.5904 mL | 33.1807 mL | 66.3614 mL | 82.9518 mL |
| 5 mM | 0.6636 mL | 3.3181 mL | 6.6361 mL | 13.2723 mL | 16.5904 mL |
| 10 mM | 0.3318 mL | 1.659 mL | 3.3181 mL | 6.6361 mL | 8.2952 mL |
| 50 mM | 0.0664 mL | 0.3318 mL | 0.6636 mL | 1.3272 mL | 1.659 mL |
| 100 mM | 0.0332 mL | 0.1659 mL | 0.3318 mL | 0.6636 mL | 0.8295 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
(-)-Indolactam V is a PKC activator, with Kis of 3.36 nM, 1.03 μM for η-CRD2 (PKCη surrogate peptide), γ-CRD2 (PKCγ surrogate peptide), and Kds of 5.5 nM (η-C1B), 7.7 nM (ε-C1B), 8.3 nM (δ-C1B), 18.9 nM (β-C1A-long), 20.8 nM (α-C1A-long), 137 nM (β-C1B), 138 nM (γ-C1A), 213 nM (γ-C1B), and has antitumor activity.
In Vitro:(-)-Indolactam V is a PKC activator, with Kis of 3.36 nM, 1.03 μM for η-CRD2 (PKCη surrogate peptide), γ-CRD2 (PKCγ surrogate peptide), and has antitumor activity[1]. (-)-Indolactam V shows Kds of 5.5 nM (η-C1B), 7.7 nM (ε-C1B), 8.3 nM (δ-C1B), 18.9 nM (β-C1A-long), 20.8 nM (α-C1A-long), 137 nM (β-C1B), 138 nM (γ-C1A), 213 nM (γ-C1B), respectively[2]. (-)-Indolactam V (20 nM-5 μM) dose-dependently affects multiple hESC lines, such as HUES 2, 4 and 8. (-)-Indolactam V also increases the mRNA levels of Pdx1, HNF6, PTF1A, SOX9, HB9 and PROX1. In addition, (-)-Indolactam V (300 nM) functions in both mouse and human cells and confirms that some signals for pancreatic development[3].
References:
[1]. Nakagawa Y, et al. Synthesis and biological activities of indolactone-V, the lactone analogue of the tumor promoter (-)-indolactam-V. Biosci Biotechnol Biochem. 1997 Aug;61(8):1415-7.
[2]. Masuda A, et al. Binding selectivity of conformationally restricted analogues of (-)-indolactam-V to the C1 domains of protein kinase C isozymes. Biosci Biotechnol Biochem. 2002 Jul;66(7):1615-7.
[3]. Chen S, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009 Apr;5(4):258-65.
- Ligularizine
Catalog No.:BCN2091
CAS No.:90364-92-4
- Neoligularidine
Catalog No.:BCN2137
CAS No.:90364-91-3
- Ligularinine
Catalog No.:BCN2117
CAS No.:90364-90-2
- Pitolisant hydrochloride
Catalog No.:BCC1863
CAS No.:903576-44-3
- Bicalutamide
Catalog No.:BCC2481
CAS No.:90357-06-5
- 7-Xylosyltaxol B
Catalog No.:BCN7675
CAS No.:90352-19-5
- 10-O-Coumaroyl-10-O-deacetylasperuloside
Catalog No.:BCN7614
CAS No.:903519-82-4
- Seneciphyllinine
Catalog No.:BCN2132
CAS No.:90341-45-0
- Shizukolidol
Catalog No.:BCN4444
CAS No.:90332-92-6
- 7-Xylosyltaxol
Catalog No.:BCN5341
CAS No.:90332-66-4
- 7-Xylosyl-10-deacetyltaxol C
Catalog No.:BCN7663
CAS No.:90332-65-3
- 7-Xylosyl-10-deacetyltaxol B
Catalog No.:BCN7667
CAS No.:90332-64-2
- Bleomycin Sulfate
Catalog No.:BCC3694
CAS No.:9041-93-4
- Neochamaejasmine B
Catalog No.:BCN3130
CAS No.:90411-12-4
- Neochamaejasmine A
Catalog No.:BCN3129
CAS No.:90411-13-5
- NU 1025
Catalog No.:BCC2454
CAS No.:90417-38-2
- Daturataturin A aglycone
Catalog No.:BCN4445
CAS No.:904665-71-0
- Daturametelin I
Catalog No.:BCN4446
CAS No.:904667-65-8
- Maoyerabdosin
Catalog No.:BCN3944
CAS No.:90468-72-7
- Valeriotetrate C
Catalog No.:BCN6753
CAS No.:904891-20-9
- Cryptochlorogenic acid
Catalog No.:BCN5907
CAS No.:905-99-7
- TMCB
Catalog No.:BCC7745
CAS No.:905105-89-7
- GDC-0879
Catalog No.:BCC2482
CAS No.:905281-76-7
- 2,5-dihydroxy-3-methoxy-Acetophenone
Catalog No.:BCN3780
CAS No.:90536-47-3
Modular Total Synthesis of Protein Kinase C Activator (-)-Indolactam V.[Pubmed:27074538]
Org Lett. 2016 May 6;18(9):2008-11.
A concise, eight-step total synthesis of (-)-Indolactam V, a nanomolar agonist of protein kinase C, is reported. The synthesis relies upon an efficient copper-catalyzed amino acid arylation to establish the indole C4-nitrogen bond. This cross-coupling method is applicable to a range of hydrophobic amino acids, providing a platform for further diversification of indolactam alkaloid scaffolds and studies on their potent biological activity.
Total syntheses of indolactam alkaloids (-)-indolactam V, (-)-pendolmycin, (-)-lyngbyatoxin A, and (-)-teleocidin A-2.[Pubmed:24839542]
Chem Sci. 2014 Jun 1;5(6):2184-2190.
We report the total syntheses of (-)-Indolactam V and the C7-substituted indolactam alkaloids (-)-pendolmycin, (-)-lyngbyatoxin A, and (-)-teleocidin A-2. The strategy for preparing indolactam V relies on a distortion-controlled indolyne functionalization reaction to establish the C4-N linkage, in addition to an intramolecular conjugate addition to build the conformationally-flexible nine-membered ring. The total synthesis of indolactam V then sets the stage for the divergent synthesis of the other targeted alkaloids. Specifically, late-stage sp(2)-sp(3) cross-couplings on an indolactam V derivative are used to introduce the key C7 substituents and the necessary quaternary carbons. These challenging couplings, in addition to other delicate manipulations, all proceed in the presence of a basic tertiary amine, an unprotected secondary amide, and an unprotected indole. Thus, our approach not only enables the enantiospecific total syntheses of four indolactam alkaloids, but also serves as a platform for probing complexity-generating and chemoselective transformations in the context of alkaloid total synthesis.
Overturning indolyne regioselectivities and synthesis of indolactam V.[Pubmed:21351773]
J Am Chem Soc. 2011 Mar 23;133(11):3832-5.
We report the design and synthesis of an indolyne that displays a reversal in regioselectivity, in both nucleophilic addition and cycloaddition reactions, compared to typical 4,5-indolynes. Our approach utilizes simple computations to predict regioselectivity in reactions of unsymmetrical arynes. With this methodology, novel benzenoid-substituted indoles can be accessed with significant regiocontrol. Furthermore, the technology provides an unconventional tactic for the synthesis of C4-substituted indole alkaloids, as demonstrated by a synthesis of indolactam V.
Synthesis of (-)-epi-indolactam V by an intramolecular Buchwald-Hartwig C-N coupling cyclization reaction.[Pubmed:23845025]
J Org Chem. 2013 Aug 2;78(15):7727-34.
The synthetic efforts toward the concise synthesis of (-)-Indolactam V from simple and commercially available starting materials using palladium- and copper-catalyzed intramolecular N-arylation strategy for the elaboration of the requisite nine-membered lactam ring as the key step are described. The incorporation of a turn-inducing structural element along the linear precursor was fundamental to achieve the heterocyclization step as well as obtain the correct regio- and chemoselectivity. The stereoselective nature in the C-N coupling cyclization reaction is interpreted in terms of minimization of allylic strain at the transition state for the palladium-amido complex formation. Meanwhile, the synthesis of the (-)-epi-indolactam V and its enantiomer have been accomplished.


