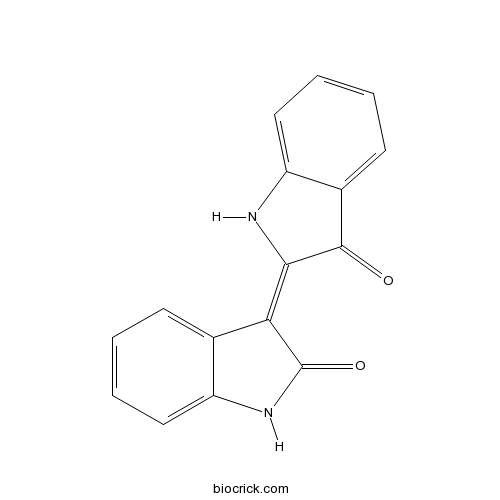
Indirubininhibitor of the production of interferon-γ CAS# 479-41-4 |
- INCB3344
Catalog No.:BCC1648
CAS No.:1262238-11-8
- RS 504393
Catalog No.:BCC1910
CAS No.:300816-15-3
- MK-0812
Catalog No.:BCC1755
CAS No.:624733-88-6
- INCB 3284 dimesylate
Catalog No.:BCC1646
CAS No.:887401-93-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 479-41-4 | SDF | Download SDF |
| PubChem ID | 5359405 | Appearance | Dark red powder |
| Formula | C16H10N2O2 | M.Wt | 262.26 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | C.I.73200; Couroupitine B; Indigo red; Indigopurpurin | ||
| Solubility | DMSO : 10 mg/mL (38.13 mM; Need ultrasonic) | ||
| Chemical Name | (3E)-3-(3-oxo-1H-indol-2-ylidene)-1H-indol-2-one | ||
| SMILES | C1=CC=C2C(=C1)C(=C3C(=O)C4=CC=CC=C4N3)C(=O)N2 | ||
| Standard InChIKey | CRDNMYFJWFXOCH-BUHFOSPRSA-N | ||
| Standard InChI | InChI=1S/C16H10N2O2/c19-15-10-6-2-4-8-12(10)17-14(15)13-9-5-1-3-7-11(9)18-16(13)20/h1-8,17H,(H,18,20)/b14-13+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Indirubin is a potent cyclin-dependent kinases and GSK-3β inhibitor with IC50 of about 5 μM and 0.6 μM. Indirubin has anticancer, anti-inflammatory,antiviral,anti-allergic contact dermatitis effects. Each indirubin derivative acts on the DNA binding of NF-Y and represses the MDR1 gene promoter with tumor cell-type specificity.Indirubin derivatives have a potential to be used as an adjunct to antiviral therapy for the treatment of severe human H5N1 disease. |
| Targets | Bcl-2/Bax | Caspase | NF-kB | IkB | MAPK | Antifection | STAT | IKK | GSK-3β | IFN-γ | IL Receptor | TNF-α | CXCL10 |
| In vitro | Enhancing effects of indirubin on the arsenic disulfide-induced apoptosis of human diffuse large B-cell lymphoma cells.[Pubmed: 25789073]Oncol Lett. 2015 Apr;9(4):1940-1946.The aim of the present study was to investigate the Indirubin-enhanced effects of arsenic disulfide (As2S2) on the proliferation and apoptosis of diffuse large B-cell lymphoma (DLBCL) cells in order to identify an optimum combination therapy.
|
| In vivo | Indirubin, a purple 3,2- bisindole, inhibited allergic contact dermatitis via regulating T helper (Th)-mediated immune system in DNCB-induced model.[Pubmed: 23149289]J Ethnopharmacol. 2013 Jan 9;145(1):214-9.Indirubin, isolated from Indigo naturalis (Apiaceae) is a purple 3,2- bisindole and a stable isomer of indigo. Although it is known to have anti-inflammatory activities, its mechanism of action has not been elucidated.
|
| Kinase Assay | Indirubin derivatives alter DNA binding activity of the transcription factor NF-Y and inhibit MDR1 gene promoter.[Pubmed: 25066113]Eur J Pharmacol. 2014 Oct 15;741:83-9.Indirubin derivatives exert antitumor activity. However, their effects on the expression of multidrug resistance gene 1 (MDR1) have not been investigated. Here we found three derivatives that inhibit the MDR1 gene promoter. |
| Cell Research | Anti-inflammatory and antiviral effects of indirubin derivatives in influenza A (H5N1) virus infected primary human peripheral blood-derived macrophages and alveolar epithelial cells.[Pubmed: 24717263]Antiviral Res. 2014 Jun;106:95-104.Human disease caused by highly pathogenic avian influenza A (HPAI) (H5N1) is associated with fulminant viral pneumonia and mortality rates in excess of 60%. Acute respiratory syndrome (ARDS) has been found to be the most severe form of acute lung injury caused by H5N1 virus infection while cytokine dysregulation and viral replication are thought to contribute to its pathogenesis.
|

Indirubin Dilution Calculator

Indirubin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.813 mL | 19.065 mL | 38.1301 mL | 76.2602 mL | 95.3252 mL |
| 5 mM | 0.7626 mL | 3.813 mL | 7.626 mL | 15.252 mL | 19.065 mL |
| 10 mM | 0.3813 mL | 1.9065 mL | 3.813 mL | 7.626 mL | 9.5325 mL |
| 50 mM | 0.0763 mL | 0.3813 mL | 0.7626 mL | 1.5252 mL | 1.9065 mL |
| 100 mM | 0.0381 mL | 0.1907 mL | 0.3813 mL | 0.7626 mL | 0.9533 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Interferon-γ is thought to be a member of the inflammatory cytokine family. Because of its augmentation of inflammatory reactions through biological activities. Indirubin, the active ingredient of Danggui Longhui Wan, has inhibitory effects on the production of interferon-γ.
In vitro: Indirubin exerted its inhibitory effects not only on interferon-γ production by human myelomonocytic HBL-38 cells but also on interferon-γ and interleukin-σ production by murine splenocytes with no influence on the proliferation of either cells [1].
In vivo: Because of Indirubin’s inhibitory activity on interferon-γ production, the authors further investigated the effects of indirubin on 2,4,6-trinitro-l-chlorobenzene-induced delayed-type hypersensitivity. When injected intraperitoneally, indirubin significantly inhibited the ear swelling of TNCB-elicited mice. Moreover,the amount of interferon-γ in the culture supernatants of elicited mouse lymphocytes was inhibited by indirubin treatment [1].
Clinical trials: Several clnical trials have been conducted to study the efficacy of indirubin for the treatments of psoriasis and Acute Promyeloid Leukemia .
Reference:
[1] Kunikata T, Tatefuji T, Aga H, Iwaki K, Ikeda M, Kurimoto M. Indirubin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur J Pharmacol. 2000 Dec 20;410(1):93-100.
- Cotoin
Catalog No.:BCN5545
CAS No.:479-21-0
- Atranorin
Catalog No.:BCN5544
CAS No.:479-20-9
- Dyphylline
Catalog No.:BCC2297
CAS No.:479-18-5
- Coumestrol
Catalog No.:BCN3949
CAS No.:479-13-0
- Calcium-Sensing Receptor Antagonists I
Catalog No.:BCC1448
CAS No.:478963-79-0
- Angiotensin 1/2 (1-6)
Catalog No.:BCC1036
CAS No.:47896-63-9
- Zopfiellamide A
Catalog No.:BCN1865
CAS No.:478945-64-1
- Kisspeptin 10 (rat)
Catalog No.:BCC6132
CAS No.:478507-53-8
- CP 339818 hydrochloride
Catalog No.:BCC7048
CAS No.:478341-55-8
- ISO-1
Catalog No.:BCC5427
CAS No.:478336-92-4
- 4-Hydroxymethylphenol 1-O-rhamnoside
Catalog No.:BCN7750
CAS No.:478314-67-9
- Gabapentin enacarbil
Catalog No.:BCC4239
CAS No.:478296-72-9
- Canthin-6-one
Catalog No.:BCN5546
CAS No.:479-43-6
- Artemetin
Catalog No.:BCN5547
CAS No.:479-90-3
- Vitexicarpin
Catalog No.:BCN5020
CAS No.:479-91-4
- Aucubin
Catalog No.:BCN5355
CAS No.:479-98-1
- [Orn8]-Urotensin II
Catalog No.:BCC5793
CAS No.:479065-85-5
- MMPIP hydrochloride
Catalog No.:BCC7528
CAS No.:479077-02-6
- TC OT 39
Catalog No.:BCC7958
CAS No.:479232-57-0
- CNQX disodium salt
Catalog No.:BCC6908
CAS No.:479347-85-8
- NBQX disodium salt
Catalog No.:BCC6907
CAS No.:479347-86-9
- AUDA
Catalog No.:BCC4023
CAS No.:479413-70-2
- 1-O-galloyl-2-O-cinnamoyl-beta-d-glucose
Catalog No.:BCC3967
CAS No.:
- CP-809101
Catalog No.:BCC1498
CAS No.:479683-64-2
Indirubin derivatives alter DNA binding activity of the transcription factor NF-Y and inhibit MDR1 gene promoter.[Pubmed:25066113]
Eur J Pharmacol. 2014 Oct 15;741:83-9.
Indirubin derivatives exert antitumor activity. However, their effects on the expression of multidrug resistance gene 1 (MDR1) have not been investigated. Here we found three derivatives that inhibit the MDR1 gene promoter. To investigate the effects of Indirubins on the DNA binding of NF-Y, a major MDR1 gene transcription factor that recognizes an inverted CCAAT element in the promoter, gel mobility shift assay was performed using the element as a probe with nuclear extracts from NG108-15, MCF7, HepG2, C2C12, and SK-N-SH cells. Among 17 compounds, 5-methoxyIndirubin inhibited the DNA binding of NF-Y significantly, whereas Indirubin-3'-oxime and 7-methoxyIndirubin 3'-oxime increased the binding considerably. After evaluating a suitable concentration of each compound for transcription analysis using living tumor cells, we performed a reporter gene assay using a reporter DNA plasmid containing EGFP cDNA fused to the MDR1 gene promoter region. Indirubin-3'-oxime exerted a significant inhibitory effect on the MDR1 promoter activity in MCF7 and HepG2 cells, and 5-methoxyIndirubin inhibited the activity only in MCF7 cells; 7-methoxyIndirubin 3'-oxime suppressed the activity in all of the cell lines. We further confirmed that the compounds reduced endogenous MDR1 transcription without any inhibitory effect on NF-Y expression. Moreover, each compound increased the doxorubicin sensitivity of MCF7 cells. These results indicate that each Indirubin derivative acts on the DNA binding of NF-Y and represses the MDR1 gene promoter with tumor cell-type specificity.
Anti-inflammatory and antiviral effects of indirubin derivatives in influenza A (H5N1) virus infected primary human peripheral blood-derived macrophages and alveolar epithelial cells.[Pubmed:24717263]
Antiviral Res. 2014 Jun;106:95-104.
Human disease caused by highly pathogenic avian influenza A (HPAI) (H5N1) is associated with fulminant viral pneumonia and mortality rates in excess of 60%. Acute respiratory syndrome (ARDS) has been found to be the most severe form of acute lung injury caused by H5N1 virus infection while cytokine dysregulation and viral replication are thought to contribute to its pathogenesis. In this study, the antiviral and anti-inflammatory effects of two Indirubin derivatives: Indirubin-3'-oxime (IM) and E804 on primary human peripherial blood-derived macrophages and type-I like pneumocytes (human alveolar epithelial cells) during influenza A (H5N1) virus infection were investigated. We found that both of the Indirubin derivatives strongly suppress the pro-inflammatory cytokines including IP-10 (CXCL10), one of the key factors which contribute to the lung inflammation during H5N1 virus infection. In addition, we also demonstrated that the Indirubin derivative delays the virus replication in the primary cell culture models. Our results showed that Indirubin derivatives have a potential to be used as an adjunct to antiviral therapy for the treatment of severe human H5N1 disease.
Enhancing effects of indirubin on the arsenic disulfide-induced apoptosis of human diffuse large B-cell lymphoma cells.[Pubmed:25789073]
Oncol Lett. 2015 Apr;9(4):1940-1946.
The aim of the present study was to investigate the Indirubin-enhanced effects of arsenic disulfide (As2S2) on the proliferation and apoptosis of diffuse large B-cell lymphoma (DLBCL) cells in order to identify an optimum combination therapy. The human DLBCL cells, LY1 and LY8, were treated with different concentrations of Indirubin for 24, 48 and 72 h. Next, the cells were treated with 10 muM As2S2 or a combination of 10 muM As2S2 and 20 muM Indirubin for 48 h. Cell proliferation inhibition was detected using cell counting kit-8 and cell apoptosis was determined using flow cytometry. The expression levels of Bcl-2, Bcl-2-associated X protein (Bax) and caspase-3 were analyzed by quantitative polymerase chain reaction (qPCR) and western blotting. The DLBCL cell viability exhibited no significant changes at 24, 48 or 72 h with increasing Indirubin concentration. In addition, the apoptotic rates of the LY1 and LY8 cells demonstrated no noticeable effects at 48 h with increasing Indirubin concentration. Following treatment with the combination of Indirubin and As2S2, the inhibitory and apoptotic rates of the cells were notably increased compared with those of the As2S2-treated group. The qPCR results revealed that Indirubin alone had no enhancing effect upon the Bax/Bcl-2 mRNA expression ratio and caspase-3 mRNA expression. Western blot analysis revealed that Indirubin alone had an enhancing effect upon the Bax/Bcl-2 protein ratio and procaspase-3 protein expression. In addition, the results demonstrated that the 21-KDa Bax protein was proteolytically cleaved into an 18-KDa Bax in the DLBCL cells treated with the combination of Indirubin and As2S2. Indirubin alone did not inhibit proliferation or induce the apoptosis of the LY1 and LY8 cells. However, the combination of Indirubin and As2S2 yielded enhancing effects. Therefore, the results of the present study demonstrated that with regard to antitumor activities, As2S2 served as the principal drug, whereas Indirubin served as the adjuvant drug. The enhancing effect was due, in part, to the induction of the mitochondrial apoptotic pathway, which involves the cleavage of Bax.
Indirubin, a purple 3,2- bisindole, inhibited allergic contact dermatitis via regulating T helper (Th)-mediated immune system in DNCB-induced model.[Pubmed:23149289]
J Ethnopharmacol. 2013 Jan 9;145(1):214-9.
ETHNOPHARMACOLOGICAL RELEVANCE: Indirubin, isolated from Indigo naturalis (Apiaceae) is a purple 3,2- bisindole and a stable isomer of indigo. Although it is known to have anti-inflammatory activities, its mechanism of action has not been elucidated. MATERIALS AND METHODS: Seven-week-old female BALB/c mice were sensitized with 1-chloro-2,4-dinitrobenzene (DNCB) to induce skin inflammation. Hematoxylin and eosin staining was performed to assess epidermal and dermal hyperplasia, which were determined by measuring the thicknesses of the epidermis and dermis, respectively. We also evaluated serum immunoglobulin E (IgE) levels and cytokines production, such as tumor necrosis factor (TNF)-alpha, interleukin (IL)-4, 6 and Interferon (IFN)-gamma. In addition, we investigated nuclear factor (NF)-kappaB, IkappaB-alpha and mitogen-activated protein (MAP) kinase activities for verifying the molecular mechanism of inflammation. RESULTS: Indirubin treatment suppressed skin inflammation in DNCB-exposed mice. The skin lesions were significantly thinner in the Indirubin-treated group than in untreated controls, and the hyperkeratosis disappeared. Indirubin reduced the total serum IgE level and cytokines production. In addition, it normalized NF-kappaB, IkappaB-alpha and MAP kinase expression. CONCLUSIONS: Indirubin might be a useful treatment for allergic contact dermatitis via regulating the co-expression of T helper (Th) 1 and 2 cell-mediated immune responses.


