ArtemetinCAS# 479-90-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 479-90-3 | SDF | Download SDF |
| PubChem ID | 5320351 | Appearance | Yellow powder |
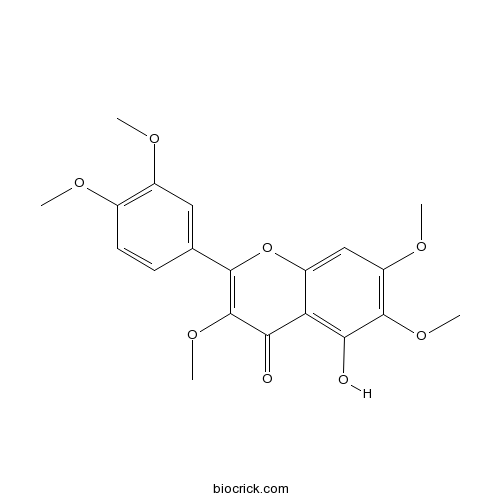
| Formula | C20H20O8 | M.Wt | 388.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Artemisetin; Erianthin; 5-Hydroxy 3,6,7,3',4'-pentamethoxyflavone; Quercetagetin 3,6,7,3',4'-pentamethyl ether | ||
| Solubility | Slightly soluble in water | ||
| Chemical Name | 2-(3,4-dimethoxyphenyl)-5-hydroxy-3,6,7-trimethoxychromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=C(C(=O)C3=C(C(=C(C=C3O2)OC)OC)O)OC)OC | ||
| Standard InChIKey | RIGYMJVFEJNCKD-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Artemetin has anti-inflammatory, antioxidant and antiapoptotic activities, it protects endothelial function through the activation of ERK1/2 and Akt. Intravenous injection of Artemetin (0.75 mg/kg) significantly reduces the hypertensive response to angiotensin I while increases the average length of bradykinin-induced hypotension. |
| Targets | NO | Estrogen receptor | PKA | NOS | Akt | ERK | p38MAPK | AChR | Progestogen receptor |
| In vitro | Effects of Artemetin on Nitric Oxide Release and Protection against Peroxidative Injuries in Porcine Coronary Artery Endothelial Cells.[Pubmed: 26032176]Phytother Res. 2015 May 29.Artemetin is one of the main components of Achillea millefolium L. and Artemisia absinthium, which have long been used for the treatment of various diseases. To date, however, available information about protective effects of their extracts on the cardiovascular system is scarce. |
| In vivo | Anti-inflammatory activity and sub-acute toxicity of artemetin.[Pubmed: 2356241]Planta Med. 1990 Feb;56(1):36-40.The 5-hydroxy-3,6,7,3',4'-pentamethoxyflavone (Artemetin) from Cordia verbenacea DC (Boraginaceae) showed marked anti-inflammatory activity using various experimental models in rats.
|
| Animal Research | Hypotensive mechanism of the extracts and artemetin isolated from Achillea millefolium L. (Asteraceae) in rats.[Pubmed: 21420289 ]Phytomedicine. 2011 Jul 15;18(10):819-25.Traditional uses of Achillea millefolium L. (Asteraceae) include the treatment of cardiovascular diseases.
|

Artemetin Dilution Calculator

Artemetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5747 mL | 12.8733 mL | 25.7467 mL | 51.4933 mL | 64.3666 mL |
| 5 mM | 0.5149 mL | 2.5747 mL | 5.1493 mL | 10.2987 mL | 12.8733 mL |
| 10 mM | 0.2575 mL | 1.2873 mL | 2.5747 mL | 5.1493 mL | 6.4367 mL |
| 50 mM | 0.0515 mL | 0.2575 mL | 0.5149 mL | 1.0299 mL | 1.2873 mL |
| 100 mM | 0.0257 mL | 0.1287 mL | 0.2575 mL | 0.5149 mL | 0.6437 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Canthin-6-one
Catalog No.:BCN5546
CAS No.:479-43-6
- Indirubin
Catalog No.:BCN2385
CAS No.:479-41-4
- Cotoin
Catalog No.:BCN5545
CAS No.:479-21-0
- Atranorin
Catalog No.:BCN5544
CAS No.:479-20-9
- Dyphylline
Catalog No.:BCC2297
CAS No.:479-18-5
- Coumestrol
Catalog No.:BCN3949
CAS No.:479-13-0
- Calcium-Sensing Receptor Antagonists I
Catalog No.:BCC1448
CAS No.:478963-79-0
- Angiotensin 1/2 (1-6)
Catalog No.:BCC1036
CAS No.:47896-63-9
- Zopfiellamide A
Catalog No.:BCN1865
CAS No.:478945-64-1
- Kisspeptin 10 (rat)
Catalog No.:BCC6132
CAS No.:478507-53-8
- CP 339818 hydrochloride
Catalog No.:BCC7048
CAS No.:478341-55-8
- ISO-1
Catalog No.:BCC5427
CAS No.:478336-92-4
- Vitexicarpin
Catalog No.:BCN5020
CAS No.:479-91-4
- Aucubin
Catalog No.:BCN5355
CAS No.:479-98-1
- [Orn8]-Urotensin II
Catalog No.:BCC5793
CAS No.:479065-85-5
- MMPIP hydrochloride
Catalog No.:BCC7528
CAS No.:479077-02-6
- TC OT 39
Catalog No.:BCC7958
CAS No.:479232-57-0
- CNQX disodium salt
Catalog No.:BCC6908
CAS No.:479347-85-8
- NBQX disodium salt
Catalog No.:BCC6907
CAS No.:479347-86-9
- AUDA
Catalog No.:BCC4023
CAS No.:479413-70-2
- 1-O-galloyl-2-O-cinnamoyl-beta-d-glucose
Catalog No.:BCC3967
CAS No.:
- CP-809101
Catalog No.:BCC1498
CAS No.:479683-64-2
- Eleutherol
Catalog No.:BCN8480
CAS No.:480-00-2
- Astragalin
Catalog No.:BCN5549
CAS No.:480-10-4
Effects of Artemetin on Nitric Oxide Release and Protection against Peroxidative Injuries in Porcine Coronary Artery Endothelial Cells.[Pubmed:26032176]
Phytother Res. 2015 Sep;29(9):1339-1348.
Artemetin is one of the main components of Achillea millefolium L. and Artemisia absinthium, which have long been used for the treatment of various diseases. To date, however, available information about protective effects of their extracts on the cardiovascular system is scarce. Therefore, we planned to analyze the effects of Artemetin on nitric oxide (NO) release and the protection exerted against oxidation in porcine aortic endothelial (PAE) cells. In PAE, we examined the modulation of NO release caused by Artemetin and the involvement of muscarinic receptors, beta2-adrenoreceptors, estrogenic receptors (ER), protein-kinase A, phospholipase-C, endothelial-NO-synthase (eNOS), Akt, extracellular-signal-regulated kinases 1/2 (ERK1/2) and p38 mitogen activated protein kinase (p38 MAPK). Moreover, in cells treated with hydrogen peroxide, the effects of Artemetin were examined on cell survival, glutathione (GSH) levels, apoptosis, mitochondrial membrane potential and transition pore opening. Artemetin increased eNOS-dependent NO production by the involvement of muscarinic receptors, beta2-adrenoreceptors, ER and all the aforementioned kinases. Furthermore, Artemetin improved cell viability in PAE that were subjected to peroxidation by counteracting GSH depletion and apoptosis and through the modulation of mitochondrial function. In conclusion, Artemetin protected endothelial function by acting as antioxidant and antiapoptotic agent and through the activation of ERK1/2 and Akt. Copyright (c) 2015 John Wiley & Sons, Ltd.
Hypotensive mechanism of the extracts and artemetin isolated from Achillea millefolium L. (Asteraceae) in rats.[Pubmed:21420289]
Phytomedicine. 2011 Jul 15;18(10):819-25.
Traditional uses of Achillea millefolium L. (Asteraceae) include the treatment of cardiovascular diseases. In the present study, we used anesthetized rats to assess the hypotensive effect of a hydroethanolic extract (HEAM), and its dichloromethane (DCM), ethyl acetate (EA), butanolic (BT), and dichloromethane-2 (DCM-2) fractions, besides the flavonoid Artemetin, isolated from A. millefolium. The oral administration of HEAM (100-300 mg/kg), DCM (20mg/kg), DCM-2 (10-30 mg/kg), but not EA (10 mg/kg) and BT (50 mg/kg) fractions significantly reduced the mean arterial pressure (MAP) of normotensive rats. The phytochemical analysis by NMR (1)H of DCM and DCM-2 fractions revealed high amounts of Artemetin, that was isolated and administered by either oral (1.5 mg/kg) or intravenous (0.15-1.5 mg/kg) routes in rats. This flavonoid was able to dose-dependently reduce the MAP, up to 11.47 +/- 1.5 mmHg (1.5 mg/kg, i.v.). To investigate if Artemetin-induced hypotension was related to angiotensin-converting enzyme inhibition, we evaluated the influence of this flavonoid on the vascular effects of both angiotensin I and bradykinin. Intravenous injection of Artemetin (0.75 mg/kg) significantly reduced the hypertensive response to angiotensin I while increased the average length of bradykinin-induced hypotension. Artemetin (1.5 mg/kg, p.o.) was also able to reduce plasma (about 37%) and vascular (up to 63%) ACE activity in vitro, compared to control group. On the other hand, Artemetin did not change angiotensin II-induced hypertension. Our study is the first showing the hypotensive effects induced by the extract and fractions obtained from A. millefollium. In addition, our results disclosed that this effect may be, at least in part, associated with high levels of Artemetin and its ability to decrease angiotensin II generation in vivo, by ACE inhibition.
Anti-inflammatory activity and sub-acute toxicity of artemetin.[Pubmed:2356241]
Planta Med. 1990 Feb;56(1):36-40.
The 5-hydroxy-3,6,7,3',4'-pentamethoxyflavone (Artemetin) from Cordia verbenacea DC (Boraginaceae) showed marked anti-inflammatory activity using various experimental models in rats. Artemetin significantly inhibited carrageenin-induced paw edema following oral doses from 30.4 to 153.9 mg.kg-1. The doses of 102.6 and 153.9 mg.kg-1 showed an inhibitory effect similar to that of 50.0 mg.kg-1 of calcium phenylbutazone. The ED50 value of Artemetin in rats was estimated to be 67.07 mg.kg-1. Repeated administration of Artemetin at doses of 67.07 mg.kg-1 for a 6-day period reduced granuloma formation with a response comparable to that of 20.0 mg.kg-1 of calcium phenylbutazone. This same dose of Artemetin also reduced the vascular permeability to intracutaneous histamine. Sub-acute toxicological experiments indicated a very low toxicity.


