Canthin-6-oneCAS# 479-43-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

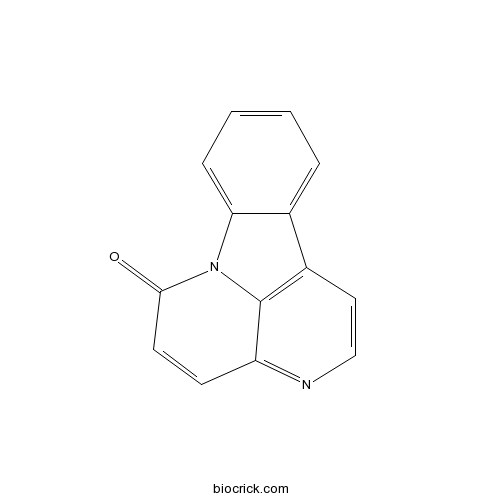
| Cas No. | 479-43-6 | SDF | Download SDF |
| PubChem ID | 97176 | Appearance | Powder |
| Formula | C14H8N2O | M.Wt | 220.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | C1=CC=C2C(=C1)C3=C4N2C(=O)C=CC4=NC=C3 | ||
| Standard InChIKey | ZERVJPYNQLONEK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H8N2O/c17-13-6-5-11-14-10(7-8-15-11)9-3-1-2-4-12(9)16(13)14/h1-8H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Canthin-6-one has antimicrobial, cytotoxic, antiproliferative and proapoptotic effects, possibly by interfering with the G2/M transition; it also has antiinflammatory activity by interfering with the transcription factors NF-κB and AP-1 at transcriptional level. |
| Targets | Caspase | Antifection | NF-κB | AP-1 |
| In vitro | Canthin-6-one alkaloids from Picrasma quassioides and their cytotoxic activity.[Pubmed: 19031238]J Asian Nat Prod Res. 2008 Nov-Dec;10(11-12):1009-12.
|
| In vivo | Pharmacological mechanisms underlying the anti-ulcer activity of methanol extract and canthin-6-one of Simaba ferruginea A. St-Hil. in animal models.[Pubmed: 21236329]J Ethnopharmacol. 2011 Apr 12;134(3):630-6.Simaba ferruginea A. St-Hil. (Simaroubaceae) is a subshrub typical of the Brazilian Cerrado, whose rhizomes are popularly used as infusion or decoction for the treatment of gastric ulcers, diarrhea and fever.
To evaluate the pharmacological mechanism(s) of action of the antiulcer effects of the methanol extract of Simaba ferruginea and its alkaloid Canthin-6-one.
|
| Kinase Assay | The MFS-type efflux pump Flr1 induced by Yap1 promotes canthin-6-one resistance in yeast.[Pubmed: 23912082]FEBS Lett. 2013 Sep 17;587(18):3045-51.
|
| Cell Research | Canthin-6-one alkaloids and a tirucallanoid from Eurycoma longifolia and their cytotoxic activity against a human HT-1080 fibrosarcoma cell line.[Pubmed: 20184012]Canthin-6-one displays antiproliferative activity and causes accumulation of cancer cells in the G2/M phase.[Pubmed: 25379743]J Nat Prod. 2014 Nov 26;77(11):2481-7.Canthinones are natural substances with a wide range of biological activities, including antipyretic, antiparasitic, and antimicrobial. Antiproliferative and/or cytotoxic effects of canthinones on cancer cells have also been described, although their mechanism of action remains ill defined. Nat Prod Commun. 2010 Jan;5(1):17-22.Phytochemical investigation of the stems of Eurycoma longifolia Jack led to the isolation of two new Canthin-6-one alkaloids, 4,9-dimethoxyCanthin-6-one (1) and 10-hydroxy-11-methoxyCanthin-6-one (2), and a new tirucallane-type triterpenoid, 23,24,25-trihydroxytirucall-7-en-3,6-dione (3), along with 37 known compounds. Among these, an oxasqualenoid (4) was isolated as a natural product for the first time. The structures of the isolates were elucidated by spectroscopic and mass spectrometric means. |

Canthin-6-one Dilution Calculator

Canthin-6-one Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5413 mL | 22.7066 mL | 45.4133 mL | 90.8265 mL | 113.5332 mL |
| 5 mM | 0.9083 mL | 4.5413 mL | 9.0827 mL | 18.1653 mL | 22.7066 mL |
| 10 mM | 0.4541 mL | 2.2707 mL | 4.5413 mL | 9.0827 mL | 11.3533 mL |
| 50 mM | 0.0908 mL | 0.4541 mL | 0.9083 mL | 1.8165 mL | 2.2707 mL |
| 100 mM | 0.0454 mL | 0.2271 mL | 0.4541 mL | 0.9083 mL | 1.1353 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Indirubin
Catalog No.:BCN2385
CAS No.:479-41-4
- Cotoin
Catalog No.:BCN5545
CAS No.:479-21-0
- Atranorin
Catalog No.:BCN5544
CAS No.:479-20-9
- Dyphylline
Catalog No.:BCC2297
CAS No.:479-18-5
- Coumestrol
Catalog No.:BCN3949
CAS No.:479-13-0
- Calcium-Sensing Receptor Antagonists I
Catalog No.:BCC1448
CAS No.:478963-79-0
- Angiotensin 1/2 (1-6)
Catalog No.:BCC1036
CAS No.:47896-63-9
- Zopfiellamide A
Catalog No.:BCN1865
CAS No.:478945-64-1
- Kisspeptin 10 (rat)
Catalog No.:BCC6132
CAS No.:478507-53-8
- CP 339818 hydrochloride
Catalog No.:BCC7048
CAS No.:478341-55-8
- ISO-1
Catalog No.:BCC5427
CAS No.:478336-92-4
- 4-Hydroxymethylphenol 1-O-rhamnoside
Catalog No.:BCN7750
CAS No.:478314-67-9
- Artemetin
Catalog No.:BCN5547
CAS No.:479-90-3
- Vitexicarpin
Catalog No.:BCN5020
CAS No.:479-91-4
- Aucubin
Catalog No.:BCN5355
CAS No.:479-98-1
- [Orn8]-Urotensin II
Catalog No.:BCC5793
CAS No.:479065-85-5
- MMPIP hydrochloride
Catalog No.:BCC7528
CAS No.:479077-02-6
- TC OT 39
Catalog No.:BCC7958
CAS No.:479232-57-0
- CNQX disodium salt
Catalog No.:BCC6908
CAS No.:479347-85-8
- NBQX disodium salt
Catalog No.:BCC6907
CAS No.:479347-86-9
- AUDA
Catalog No.:BCC4023
CAS No.:479413-70-2
- 1-O-galloyl-2-O-cinnamoyl-beta-d-glucose
Catalog No.:BCC3967
CAS No.:
- CP-809101
Catalog No.:BCC1498
CAS No.:479683-64-2
- Eleutherol
Catalog No.:BCN8480
CAS No.:480-00-2
Pharmacological mechanisms underlying the anti-ulcer activity of methanol extract and canthin-6-one of Simaba ferruginea A. St-Hil. in animal models.[Pubmed:21236329]
J Ethnopharmacol. 2011 Apr 12;134(3):630-6.
RELEVANCE: Simaba ferruginea A. St-Hil. (Simaroubaceae) is a subshrub typical of the Brazilian Cerrado, whose rhizomes are popularly used as infusion or decoction for the treatment of gastric ulcers, diarrhea and fever. AIM OF THE STUDY: To evaluate the pharmacological mechanism(s) of action of the antiulcer effects of the methanol extract of Simaba ferruginea and its alkaloid Canthin-6-one. MATERIALS AND METHODS: Rhizome of Simaba ferruginea was macerated with methanol to obtain the methanol extract (MESf) from which was obtained, the chloroform fraction. Canthin-6-one alkaloid (Cant) was purified and then isolated from the chloroform fraction (CFSf). The isolated Cant was identified by HPLC. Anti-ulcer assays were determined using ethanol and indomethacin-induced ulcer models in mice and rats respectively. In order to determine the probable mechanisms of actions of MESf and Cant animals were pretreated with l-NAME prior to anti-ulcer agent treatments and ulcer induction and nitric oxide (NO) level determined in order to assess NO involvement in the gastroprotective effects. Assays of malondialdehyde (MDA), myeloperoxidase (MPO), pro-inflammatory cytokines: interleukin 8 (IL-8) and tumor necrosis factor-alpha (TNF-alpha) and prostaglandin E(2) (PGE(2)) were also carried out according to previously described methods. RESULTS: The results indicate that the antiulcerogenic effects of MESf and Cant in ethanol-induced ulcer is mediated in part through increase in the production of protective endogenous NO as the antiulcerogenic activity of MESf and Cant was reduced in animals pre-treated with l-NAME. In indomethacin-induced ulcer pre-treatment with MESf and Cant showed reduction in the levels of MPO and MDA in the gastric tissue, thus indicating the participation of the antioxidant mechanisms on the gastroprotective effects. The plasma levels of IL-8 in ulcerated rats with indomethacin were also reduced by Cant, but not by MESf, indicating that inhibition of this cytokine contributes to the gastroprotective effect of Cant. However MESf and Cant had no effect on the mucosal membrane levels of PGE(2), indicating that the gastroprotective effects of these agents is independent of PGE(2) modulation. CONCLUSION: The results obtained in this study with MESf and Cant added insights into the pharmacological mechanisms involved in their mode of antiulcer action. The results indicate that Cant is one of the compounds responsible for these effects. Such findings are of extreme importance in the strive for future development of potent, safer and effective antiulcer agent. The efficacy of MESf and Cant in gastroprotection shows that Simaba ferruginea might be a promising antiulcer herbal medicine, in addition to confirming the popular use of this plant against gastric ulcer models utilised in this study.
Canthin-6-one alkaloids from Picrasma quassioides and their cytotoxic activity.[Pubmed:19031238]
J Asian Nat Prod Res. 2008 Nov-Dec;10(11-12):1009-12.
A new alkaloid, 4,5-dimethoxy-10-hydroxyCanthin-6-one (1), was isolated from the stem of Picrasma quassioides Bennet (Simaroubaceae) together with four known Canthin-6-one alkaloids, 8-hydroxyCanthin-6-one (2), 4,5-dimethoxyCanthin-6-one (3), 5-hydroxy-4-methoxyCanthin-6-one (4), and 3-methylcanthin-5,6-dione (5). Their structures were elucidated on the basis of spectroscopic data. The cytotoxic activity of the Canthin-6-one alkaloids was evaluated using human nasopharyngeal carcinoma (CNE2) and human liver cancer (Bel-7402) cell lines. Among these isolates, compounds 1-4 exhibited significant cytotoxic activity against CNE2 cell line.
The MFS-type efflux pump Flr1 induced by Yap1 promotes canthin-6-one resistance in yeast.[Pubmed:23912082]
FEBS Lett. 2013 Sep 17;587(18):3045-51.
Screening for suppressors of Canthin-6-one toxicity in yeast identified Yap1, a transcription factor involved in cell response to a broad range of injuries. Although Canthin-6-one did not promote a significant oxidative stress, overexpression of YAP1 gene clearly increased resistance to this drug. We demonstrated that Yap1-mediated resistance involves the plasma membrane major-facilitator-superfamily efflux pump Flr1 but not the vacuolar ATP-binding-cassette transporter Ycf1. FLR1 overexpression was sufficient to reduce sensitivity to the drug, but strictly dependent on a functional YAP1 gene.
Canthin-6-one alkaloids and a tirucallanoid from Eurycoma longifolia and their cytotoxic activity against a human HT-1080 fibrosarcoma cell line.[Pubmed:20184012]
Nat Prod Commun. 2010 Jan;5(1):17-22.
Phytochemical investigation of the stems of Eurycoma longifolia Jack led to the isolation of two new Canthin-6-one alkaloids, 4,9-dimethoxyCanthin-6-one (1) and 10-hydroxy-11-methoxyCanthin-6-one (2), and a new tirucallane-type triterpenoid, 23,24,25-trihydroxytirucall-7-en-3,6-dione (3), along with 37 known compounds. Among these, an oxasqualenoid (4) was isolated as a natural product for the first time. The structures of the isolates were elucidated by spectroscopic and mass spectrometric means. All the isolates were evaluated for their cytotoxic activity against a HT-1080 human fibrosarcoma cell line. Among them, 9,10-dimethoxyCanthin-6-one (14, IC50 = 5.0 microM), 10-hydroxy-9-methoxyCanthin-6-one (15, IC50 = 7.2 microM), dihydroniloticin (18, IC50 = 8.2 microM), and 14-deacetyleurylene (34, IC50 = 3.2 microM) displayed stronger activity than the positive control 5-FU (IC50 = 9.2 microM).
Canthin-6-one displays antiproliferative activity and causes accumulation of cancer cells in the G2/M phase.[Pubmed:25379743]
J Nat Prod. 2014 Nov 26;77(11):2481-7.
Canthinones are natural substances with a wide range of biological activities, including antipyretic, antiparasitic, and antimicrobial. Antiproliferative and/or cytotoxic effects of canthinones on cancer cells have also been described, although their mechanism of action remains ill defined. To gain better insight into this mechanism, the antiproliferative effect of a commercially available Canthin-6-one (1) was examined dose-dependently on six cancer cell lines (human prostate, PC-3; human colon, HT-29; human lymphocyte, Jurkat; human cervix, HeLa; rat glioma, C6; and mouse embryonic fibroblasts, NIH-3T3). Cytotoxic effects of 1 were investigated on the same cancer cell lines by procaspase-3 cleavage and on normal human skin fibroblasts. Strong antiproliferative effects of the compound were observed in all cell lines, whereas cytotoxic effects were very dependent on cell type. A better definition of the mechanism of action of 1 was obtained on PC-3 cells, by showing that it decreases BrdU incorporation into DNA by 60% to 80% and mitotic spindle formation by 70% and that it causes a 2-fold accumulation of cells in the G2/M phase of the cell cycle. Together, the data suggest that the primary effect of Canthin-6-one (1) is antiproliferative, possibly by interfering with the G2/M transition. Proapoptotic effects might result from this disturbance of the cell cycle.


