Hexanorcucurbitacin DCAS# 29065-05-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29065-05-2 | SDF | Download SDF |
| PubChem ID | 45272227 | Appearance | Powder |
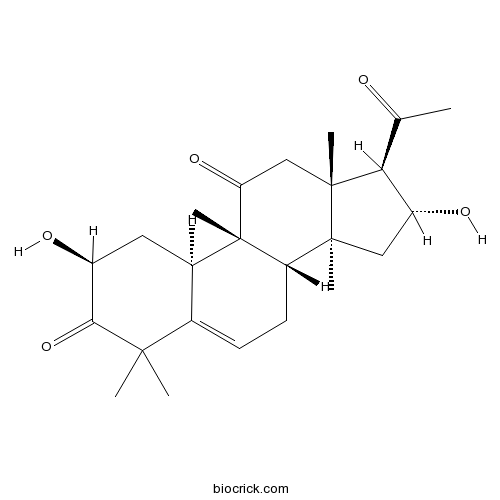
| Formula | C24H34O5 | M.Wt | 402.52 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,8S,9R,10R,13R,14S,16R,17R)-17-acetyl-2,16-dihydroxy-4,4,9,13,14-pentamethyl-2,7,8,10,12,15,16,17-octahydro-1H-cyclopenta[a]phenanthrene-3,11-dione | ||
| SMILES | CC(=O)C1C(CC2(C1(CC(=O)C3(C2CC=C4C3CC(C(=O)C4(C)C)O)C)C)C)O | ||
| Standard InChIKey | DBGQLXXAKKEGFX-KFUQAJKQSA-N | ||
| Standard InChI | InChI=1S/C24H34O5/c1-12(25)19-16(27)10-22(4)17-8-7-13-14(9-15(26)20(29)21(13,2)3)24(17,6)18(28)11-23(19,22)5/h7,14-17,19,26-27H,8-11H2,1-6H3/t14-,15+,16-,17+,19+,22+,23-,24+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Hexanorcucurbitacin D is a weak agonist acting at the ecdysteroid receptor. |

Hexanorcucurbitacin D Dilution Calculator

Hexanorcucurbitacin D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4843 mL | 12.4217 mL | 24.8435 mL | 49.687 mL | 62.1087 mL |
| 5 mM | 0.4969 mL | 2.4843 mL | 4.9687 mL | 9.9374 mL | 12.4217 mL |
| 10 mM | 0.2484 mL | 1.2422 mL | 2.4843 mL | 4.9687 mL | 6.2109 mL |
| 50 mM | 0.0497 mL | 0.2484 mL | 0.4969 mL | 0.9937 mL | 1.2422 mL |
| 100 mM | 0.0248 mL | 0.1242 mL | 0.2484 mL | 0.4969 mL | 0.6211 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Matricin
Catalog No.:BCC8209
CAS No.:29041-35-8
- Bis(2-carboxyethyl)isocyanurate
Catalog No.:BCC8880
CAS No.:2904-40-7
- BMS 299897
Catalog No.:BCC2340
CAS No.:290315-45-6
- Glucosamine sulfate
Catalog No.:BCN5981
CAS No.:29031-19-4
- Daun02
Catalog No.:BCC1518
CAS No.:290304-24-4
- 3-Epiglochidiol
Catalog No.:BCN5193
CAS No.:29028-10-2
- Fmoc-Gly-OH
Catalog No.:BCC3498
CAS No.:29022-11-5
- Benzoyl-DL-valine
Catalog No.:BCC8864
CAS No.:2901-80-6
- Methyl reserpate
Catalog No.:BCN3489
CAS No.:2901-66-8
- Boc-Phg-OH
Catalog No.:BCC3311
CAS No.:2900-27-8
- TRAM-34
Catalog No.:BCC1122
CAS No.:289905-88-0
- 3-(Benzylthio)-Propionic acid
Catalog No.:BCC2839
CAS No.:2899-66-3
- Pachymic acid
Catalog No.:BCN6347
CAS No.:29070-92-6
- 5-Hydroxy-3',4',7-trimethoxyflavone
Catalog No.:BCN5194
CAS No.:29080-58-8
- Eupteleasaponin I
Catalog No.:BCN7839
CAS No.:290809-29-9
- Avosentan
Catalog No.:BCC1387
CAS No.:290815-26-8
- Cimiracemoside D
Catalog No.:BCN2789
CAS No.:290821-39-5
- Glipizide
Catalog No.:BCC3785
CAS No.:29094-61-9
- Pinoresinol dimethyl ether
Catalog No.:BCN6767
CAS No.:29106-36-3
- Procyanidin B2
Catalog No.:BCN6315
CAS No.:29106-49-8
- Guanfacine
Catalog No.:BCC5180
CAS No.:29110-47-2
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- (+)-Affinisine
Catalog No.:BCN3520
CAS No.:2912-11-0
- 1-Methoxyberberine
Catalog No.:BCN7373
CAS No.:29133-52-6
Cucurbitane-type triterpenes with anti-proliferative effects on U937 cells from an egyptian natural medicine, Bryonia cretica: structures of new triterpene glycosides, bryoniaosides A and B.[Pubmed:20460809]
Chem Pharm Bull (Tokyo). 2010 May;58(5):747-51.
The 90% aqueous ethanol extract of an Egyptian natural medicine, the roots of Bryonia cretica L., was found to exhibit a strong inhibitory effect on the proliferation of human leukemia U937 cells. By bioassay-guided fractionation, we isolated two new cucurbitane-type triterpene glycosides, bryoniaosides A and B, were isolated from the roots of Bryonia cretica L. together with 16 known cucurbitane-type triterpenes and glycosides. The chemical structures of bryoniaosides A and B were determined on the basis of chemical and spectroscopic evidence. Effects of principal cucurbitane-type triterpenes (cucurbitacins B, D, E, and J, 23,24-dihydrocucurbitacins B and E, and Hexanorcucurbitacin D) on proliferation of the cells were examined. Cucurbitacins B and E showed the greater cytotoxic effects with IC(50) values of 9.2 and 16 nM after 72 h, and their IC(50) values were equivalent to that of camptothecin. An alpha,beta-conjugated ketone moiety at the 22-24-positions and an acetoxy group at the 25-position are essential for the strong activity.
Cucurbitacins are insect steroid hormone antagonists acting at the ecdysteroid receptor.[Pubmed:9581538]
Biochem J. 1997 Nov 1;327 ( Pt 3):643-50.
Two triterpenoids, cucurbitacins B and D, have been isolated from seeds of Iberis umbellata (Cruciferae) and shown to be responsible for the antagonistic activity of a methanolic extract of this species in preventing the 20-hydroxyecdysone (20E)-induced morphological changes in the Drosophila melanogaster BII permanent cell line. With a 20E concentration of 50 nM, cucurbitacins B and D give 50% responses at 1.5 and 10 microM respectively. Both cucurbitacins are able to displace specifically bound radiolabelled 25-deoxy-20-hydroxyecdysone (ponasterone A) from a cell-free preparation of the BII cells containing ecdysteroid receptors. The Kd values for cucurbitacins B and D (5 and 50 microM respectively) are similar to the concentrations required to antagonize 20E activity with whole cells. Cucurbitacin B (cucB) prevents stimulation by 20E of an ecdysteroid-responsive reporter gene in a transfection assay. CucB also prevents the formation of the Drosophila ecdysteroid receptor/Ultraspiracle/20E complex with the hsp27 ecdysteroid response element as demonstrated by gel-shift assay. This is therefore the first definitive evidence for the existence of antagonists acting at the ecdysteroid receptor. Preliminary structure/activity studies indicate the importance of the Delta23-22-oxo functional grouping in the side chain for antagonistic activity. Hexanorcucurbitacin D, which lacks carbon atoms C-22 to C-27, is found to be a weak agonist rather than an antagonist. Moreover, the side chain analogue 5-methylhex-3-en-2-one possesses weak antagonistic activity.


