MatricinCAS# 29041-35-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29041-35-8 | SDF | Download SDF |
| PubChem ID | 92265 | Appearance | Powder |
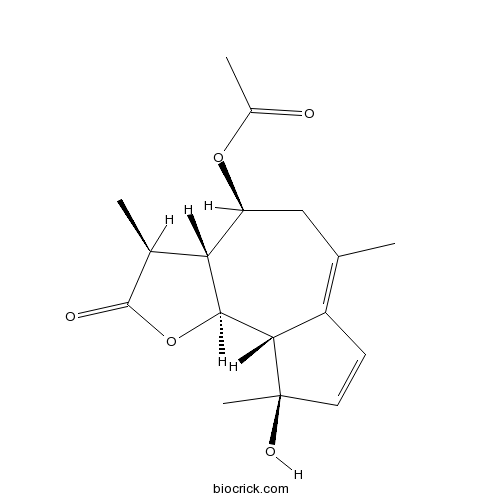
| Formula | C17H22O5 | M.Wt | 306.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(3S,3aR,4S,9R,9aS,9bS)-9-hydroxy-3,6,9-trimethyl-2-oxo-3,3a,4,5,9a,9b-hexahydroazuleno[4,5-b]furan-4-yl] acetate | ||
| SMILES | CC1C2C(CC(=C3C=CC(C3C2OC1=O)(C)O)C)OC(=O)C | ||
| Standard InChIKey | SYTRJRUSWMMZLV-VQGWEXQJSA-N | ||
| Standard InChI | InChI=1S/C17H22O5/c1-8-7-12(21-10(3)18)13-9(2)16(19)22-15(13)14-11(8)5-6-17(14,4)20/h5-6,9,12-15,20H,7H2,1-4H3/t9-,12-,13+,14-,15-,17+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Matricin Dilution Calculator

Matricin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2637 mL | 16.3185 mL | 32.6371 mL | 65.2742 mL | 81.5927 mL |
| 5 mM | 0.6527 mL | 3.2637 mL | 6.5274 mL | 13.0548 mL | 16.3185 mL |
| 10 mM | 0.3264 mL | 1.6319 mL | 3.2637 mL | 6.5274 mL | 8.1593 mL |
| 50 mM | 0.0653 mL | 0.3264 mL | 0.6527 mL | 1.3055 mL | 1.6319 mL |
| 100 mM | 0.0326 mL | 0.1632 mL | 0.3264 mL | 0.6527 mL | 0.8159 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bis(2-carboxyethyl)isocyanurate
Catalog No.:BCC8880
CAS No.:2904-40-7
- BMS 299897
Catalog No.:BCC2340
CAS No.:290315-45-6
- Glucosamine sulfate
Catalog No.:BCN5981
CAS No.:29031-19-4
- Daun02
Catalog No.:BCC1518
CAS No.:290304-24-4
- 3-Epiglochidiol
Catalog No.:BCN5193
CAS No.:29028-10-2
- Fmoc-Gly-OH
Catalog No.:BCC3498
CAS No.:29022-11-5
- Benzoyl-DL-valine
Catalog No.:BCC8864
CAS No.:2901-80-6
- Methyl reserpate
Catalog No.:BCN3489
CAS No.:2901-66-8
- Boc-Phg-OH
Catalog No.:BCC3311
CAS No.:2900-27-8
- TRAM-34
Catalog No.:BCC1122
CAS No.:289905-88-0
- 3-(Benzylthio)-Propionic acid
Catalog No.:BCC2839
CAS No.:2899-66-3
- H-Methioninol
Catalog No.:BCC2721
CAS No.:2899-37-8
- Hexanorcucurbitacin D
Catalog No.:BCN7875
CAS No.:29065-05-2
- Pachymic acid
Catalog No.:BCN6347
CAS No.:29070-92-6
- 5-Hydroxy-3',4',7-trimethoxyflavone
Catalog No.:BCN5194
CAS No.:29080-58-8
- Eupteleasaponin I
Catalog No.:BCN7839
CAS No.:290809-29-9
- Avosentan
Catalog No.:BCC1387
CAS No.:290815-26-8
- Cimiracemoside D
Catalog No.:BCN2789
CAS No.:290821-39-5
- Glipizide
Catalog No.:BCC3785
CAS No.:29094-61-9
- Pinoresinol dimethyl ether
Catalog No.:BCN6767
CAS No.:29106-36-3
- Procyanidin B2
Catalog No.:BCN6315
CAS No.:29106-49-8
- Guanfacine
Catalog No.:BCC5180
CAS No.:29110-47-2
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- (+)-Affinisine
Catalog No.:BCN3520
CAS No.:2912-11-0
Chamazulene: an antioxidant-type inhibitor of leukotriene B4 formation.[Pubmed:7997466]
Planta Med. 1994 Oct;60(5):410-3.
Matricine and its transformation product chamazulene are constituents of chamomile extracts. Both have been demonstrated to exert anti-inflammatory activity in vivo. Since preparations from chamomile are used for the treatment of inflammatory skin and bowel diseases, we studied the effects of these compounds on the leukotriene production in neutrophilic granulocytes. Chamazulene inhibited the formation of leukotriene B4 in intact cells and in the 105,000 x g supernatant fraction in a concentration-dependent manner. The IC50 values were 15 and 10 microM, respectively. Matricine showed no effect up to 200 microM. Chamazulene (IC50: 2 microM), but not Matricine, blocked the chemical peroxidation of arachidonic acid. Additionally, Matricine (up to 200 microM) had no effects on the cyclooxygenase and 12-lipoxygenase activities in human platelets. Therefore, it is concluded that chamazulene, but not Matricine, may contribute to the anti-inflammatory activity of chamomile extracts by inhibiting the leukotriene synthesis and additional antioxidative effects.
Chamazulene carboxylic acid and matricin: a natural profen and its natural prodrug, identified through similarity to synthetic drug substances.[Pubmed:16872141]
J Nat Prod. 2006 Jul;69(7):1041-5.
Chamazulene carboxylic acid (1) is a natural profen with anti-inflammatory activity and a degradation product of proazulenic sesquiterpene lactones, e.g., Matricin. Both 1 and proazulenes occur in chamomile (Matricaria recutita), yarrow (Achillea millefolium), and a few other Asteraceae species. It was isolated in improved yields, characterized physicochemically, and found to be an inhibitor of cyclooxygenase-2, but not of cyclooxygenase-1. It had anti-inflammatory activity in several animal models with local and systemic application. When human volunteers were given Matricin orally, plasma levels of 1 were found to be in the micromolar range. Matricin was converted to 1 in artificial gastric fluid, but not in artificial intestinal fluid. Matricin and the yarrow proazulenes are proposed to be anti-inflammatory through conversion to 1. Intriguingly, the biological activity of the natural compound 1 was found because of its similarity to fully synthetic drug substances. This is the reverse process of the common lead function of natural compounds in drug discovery.
Supercritical carbon dioxide extraction of chamomile flowers: extraction efficiency, stability, and in-line inclusion of chamomile-carbon dioxide extract in beta-cyclodextrin.[Pubmed:15311845]
Phytochem Anal. 2004 Jul-Aug;15(4):249-56.
The extraction of chamomile flowers using supercritical carbon dioxide was investigated with respect to extraction efficiency and compared with solvent extraction. The stability of Matricine, a sensitive constituent of the essential oil of chamomile, in these extracts was studied during storage at different temperatures over 6 months. Matricine was stable at -30 degrees C. A slight decrease (80-90% recovery) occurred at +5 degrees C, whereas complete decomposition of Matricine took place within 3-4 months at room temperature and at +30 degrees C, respectively. An in-line inclusion of chamomile constituents in beta-cyclodextrin (beta-CD) during the extraction process was assessed and inclusion rates between 40 and 95% were obtained depending on the amount of beta-CD and the type of chamomile constituent. No further stabilization of Matricine in the carbon dioxide extract/beta-CD complexes was achieved. High residual water contents in the complexes even after freeze-drying were identified as accelerating the decomposition. In addition, the extractability of flavonoids, such as apigenin and apigenin-7-glucoside, was determined. Apigenin-7-glucoside, the more hydrophilic substance, was not extractable with pure carbon dioxide and showed a recovery of 11% using methanol modified carbon dioxide (18%, w/w) at 60 degrees C and 380 bar. Extraction conditions in the two-phase region of the binary mixture carbon dioxide-methanol (70 degrees C, 100 bar) led to a drastic change in fluid polarity and hence extractability increased to 92-95%.


