GriffithinamCAS# 240122-32-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 240122-32-1 | SDF | Download SDF |
| PubChem ID | 5317823 | Appearance | Yellow powder |
| Formula | C17H13NO4 | M.Wt | 295.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
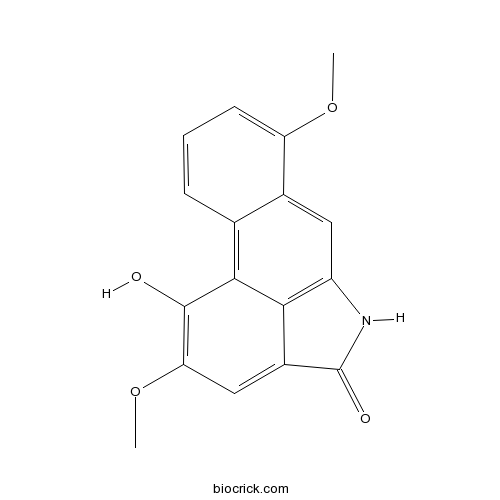
| SMILES | COC1=CC=CC2=C3C4=C(C=C21)NC(=O)C4=CC(=C3O)OC | ||
| Standard InChIKey | QKAHURDEAZTVNH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H13NO4/c1-21-12-5-3-4-8-9(12)6-11-14-10(17(20)18-11)7-13(22-2)16(19)15(8)14/h3-7,19H,1-2H3,(H,18,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | Fitoterapia. 2011 Oct;82(7):964-8.Cytotoxic alkaloids from stems, leaves and twigs of Dasymaschalon blumei.[Pubmed: 21641972]
J Nat Prod. 1999 Jul;62(7):1050-2.Alkaloids from the roots of goniothalamus griffithii.[Pubmed: 10425141]
|

Griffithinam Dilution Calculator

Griffithinam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3864 mL | 16.9319 mL | 33.8639 mL | 67.7277 mL | 84.6597 mL |
| 5 mM | 0.6773 mL | 3.3864 mL | 6.7728 mL | 13.5455 mL | 16.9319 mL |
| 10 mM | 0.3386 mL | 1.6932 mL | 3.3864 mL | 6.7728 mL | 8.466 mL |
| 50 mM | 0.0677 mL | 0.3386 mL | 0.6773 mL | 1.3546 mL | 1.6932 mL |
| 100 mM | 0.0339 mL | 0.1693 mL | 0.3386 mL | 0.6773 mL | 0.8466 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Griffithazanone A
Catalog No.:BCN4813
CAS No.:240122-30-9
- QX 314 bromide
Catalog No.:BCC6889
CAS No.:24003-58-5
- Pyrocatechol monoglucoside
Catalog No.:BCN4667
CAS No.:2400-71-7
- Dehydroepiandrosterone enanthate
Catalog No.:BCC8930
CAS No.:23983-43-9
- 13-Deacetyltaxachitriene A
Catalog No.:BCN7390
CAS No.:239800-99-8
- Tolnaftate
Catalog No.:BCC4869
CAS No.:2398-96-1
- Delta-9-Tetrahydrocannabinolic acid
Catalog No.:BCN8098
CAS No.:23978-85-0
- Meranzin
Catalog No.:BCN5092
CAS No.:23971-42-8
- 4,4'-Bis(5-methyl-2-benzoxazolyl)stilbene
Catalog No.:BCC8657
CAS No.:2397-00-4
- Glochidonol
Catalog No.:BCN5091
CAS No.:23963-54-4
- 5-[(2R)-2-Aminopropyl]-1-[3-(benzoyloxy)propyl]-2,3-dihydro-1H-indole-7-carbonitrile (2R,3R)-2,3-dihydroxybutanedioate
Catalog No.:BCN1479
CAS No.:239463-85-5
- 5-(2-Aminopropyl)-7-cyanoindolin-1-yl)propyl benzoate
Catalog No.:BCC8718
CAS No.:239463-72-0
- 2-Amino-3-benzyloxypyridine
Catalog No.:BCC8524
CAS No.:24016-03-3
- Agathadiol diacetate
Catalog No.:BCN5093
CAS No.:24022-13-7
- H-Glu-pNA
Catalog No.:BCC2923
CAS No.:24032-35-7
- Salvicine
Catalog No.:BCN3163
CAS No.:240423-23-8
- Isocurcumenol
Catalog No.:BCN3526
CAS No.:24063-71-6
- 5-Chlorothiophene-2-carboxylic acid
Catalog No.:BCC8745
CAS No.:24065-33-6
- Digiferruginol
Catalog No.:BCN3450
CAS No.:24094-45-9
- 6-Isopentenyloxyisobergapten
Catalog No.:BCC8110
CAS No.:24099-29-4
- Sibiricose A6
Catalog No.:BCN2786
CAS No.:241125-75-7
- Sibiricaxanthone B
Catalog No.:BCN2784
CAS No.:241125-81-5
- Adaphostin
Catalog No.:BCC3890
CAS No.:241127-58-2
- Flupenthixol dihydrochloride
Catalog No.:BCC7851
CAS No.:2413-38-9
Alkaloids from the roots of goniothalamus griffithii[Pubmed:10425141]
J Nat Prod. 1999 Jul;62(7):1050-2.
Three new alkaloids-griffithazanone A (1), griffithdione (2), and Griffithinam (3)-were isolated from the roots of Goniothalamus griffithii, along with six known compounds, 4-methyl-2,9, 10-(2H)-1-azaanthracenetrione (4), velutinam, aristololactam BI, aristololactam BII, aristololactam AII, and norcepharanone B. Their structures were elucidated on the basis of spectral and chemical methods. The absolute configuration of griffithazanone A (1) was determined by the preparation of Mosher's esters.
Cytotoxic alkaloids from stems, leaves and twigs of Dasymaschalon blumei.[Pubmed:21641972]
Fitoterapia. 2011 Oct;82(7):964-8.
Bioassay-guided fractionation of the cytotoxic ethyl acetate extract from the stems of Dasymaschalon blumei (Annonaceae) led to the isolation of four aristololactam alkaloids, including the hitherto unknown 3,5-dihydroxy-2,4-dimethoxyaristolactam (1), as well as the three known compounds, aristolactam BI, goniopedaline, and Griffithinam. Additionally, the cytotoxic extract from the combined leaves and twigs of the same plant yielded three known oxoaporphine alkaloids, oxodiscoguattine, dicentrinone, and duguevalline. The structures of aristolactams and oxoaporphine alkaloids were elucidated on the basis of spectroscopic methods. All isolates were evaluated for cytotoxicity against a panel of mammalian cancer cell lines and a noncancerous human embryonic kidney cell Hek 293.


