GlycopyrrolateCAS# 596-51-0 |
- Betamethasone
Catalog No.:BCC4765
CAS No.:378-44-9
- Beclomethasone dipropionate
Catalog No.:BCC4257
CAS No.:5534-09-8
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
- Mifepristone
Catalog No.:BCC4486
CAS No.:84371-65-3
- Betamethasone hydrochloride
Catalog No.:BCC4256
CAS No.:956901-32-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 596-51-0 | SDF | Download SDF |
| PubChem ID | 11693 | Appearance | Powder |
| Formula | C19H28BrNO3 | M.Wt | 398.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Glycopyrrolate bromide; Glycopyrronium bromide | ||
| Solubility | H2O : ≥ 45 mg/mL (112.97 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (1,1-dimethylpyrrolidin-1-ium-3-yl) 2-cyclopentyl-2-hydroxy-2-phenylacetate;bromide | ||
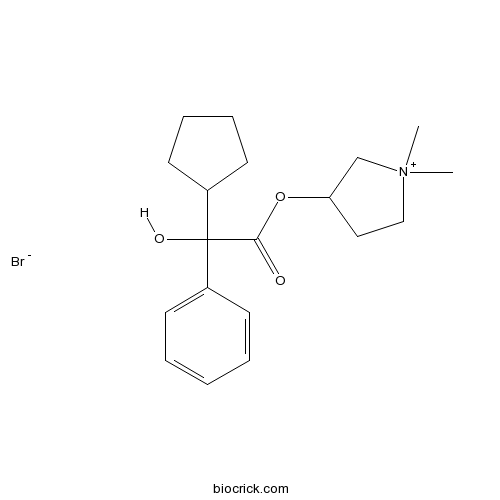
| SMILES | C[N+]1(CCC(C1)OC(=O)C(C2CCCC2)(C3=CC=CC=C3)O)C.[Br-] | ||
| Standard InChIKey | VPNYRYCIDCJBOM-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C19H28NO3.BrH/c1-20(2)13-12-17(14-20)23-18(21)19(22,16-10-6-7-11-16)15-8-4-3-5-9-15;/h3-5,8-9,16-17,22H,6-7,10-14H2,1-2H3;1H/q+1;/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glycopyrrolate(Glycopyrronium Br) is a muscarinic competitive antagonist used as an antispasmodic.
IC50 Value:
Target: mAChR (Muscarinic acetylcholine receptor M1)
in vitro: Glycopyrrolate showed no selectivity in its binding to the M1-M3 receptors. Kinetics studies, however, showed that glycopyrrolate dissociates slowly from HASM muscarinic receptors (60% protection against [3H]-NMS binding at 30 nM) compared to ipratropium bromide [1].
in vivo: Glycopyrrolate (1 mg) tablets were then administered, starting with one tablet daily the third week and increasing the daily dose by one tablet per week until a maximum of four tablets during week six and 4 days of week seven when the daily dose was reduced to two tablets for 3 days. glycopyrrolate can be given in controlled doses provided that an adequate medical assessment has been undertaken [2]. Glycopyrrolate has a slow and erratic absorption from the gastrointestinal system, but even low plasma levels are associated with a distinct and long-lasting antisialogic effect [3]. Oral glycopyrrolate is emerging as a potential second-line treatment option, but experience with safety, efficacy, and dosing is especially limited in children [4]. phase III study, 52.3% of glycopyrrolate oral solution recipients (aged 3-18 years; n = 137) had an mTDS response (primary endpoint); the response rate was consistently above 50% at all 4-weekly timepoints, aside from the first assessment at week 4 (40.3%). In general, glycopyrrolate oral solution was well tolerated in clinical trials. The majority of adverse events were within expectations as characteristic anticholinergic outcomes [5].
Toxicity: Side effects include dry mouth, difficult urinating, heachaches, diarrhea and constipation. The medication also induces drowsiness or blurred vision. LD50=709 mg/kg (rat, oral). References: | |||||

Glycopyrrolate Dilution Calculator

Glycopyrrolate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5105 mL | 12.5524 mL | 25.1048 mL | 50.2096 mL | 62.762 mL |
| 5 mM | 0.5021 mL | 2.5105 mL | 5.021 mL | 10.0419 mL | 12.5524 mL |
| 10 mM | 0.251 mL | 1.2552 mL | 2.5105 mL | 5.021 mL | 6.2762 mL |
| 50 mM | 0.0502 mL | 0.251 mL | 0.5021 mL | 1.0042 mL | 1.2552 mL |
| 100 mM | 0.0251 mL | 0.1255 mL | 0.251 mL | 0.5021 mL | 0.6276 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Glycopyrrolate is a muscarinic competitive antagonist used as an antispasmodic.
- H-D-Gln-OH
Catalog No.:BCC2920
CAS No.:5959-95-5
- 3-Amino-2-naphthoic acid
Catalog No.:BCC8607
CAS No.:5959-52-4
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- H-D-Ala-OtBu.HCl
Catalog No.:BCC2849
CAS No.:59531-86-1
- Boc-Ser-OBzl
Catalog No.:BCC3440
CAS No.:59524-02-6
- Megestrol Acetate
Catalog No.:BCC4365
CAS No.:595-33-5
- Soyasapogenol B
Catalog No.:BCN4097
CAS No.:595-15-3
- Calycanthine
Catalog No.:BCN7823
CAS No.:595-05-1
- Testosterone undecanoate
Catalog No.:BCC9173
CAS No.:5949-44-0
- Citric acid monohydrate
Catalog No.:BCN8492
CAS No.:5949-29-1
- Z-D-Glu(OBzl)-OH
Catalog No.:BCC2772
CAS No.:59486-73-6
- Tafamidis
Catalog No.:BCC5268
CAS No.:594839-88-0
- Alpha-Obscurine
Catalog No.:BCN6701
CAS No.:596-55-4
- AC 55649
Catalog No.:BCC7359
CAS No.:59662-49-6
- Calyciphylline A
Catalog No.:BCN4098
CAS No.:596799-30-3
- 3-O-Acetyl-beta-boswellic acid
Catalog No.:BCN2672
CAS No.:5968-70-7
- 6alpha-Hydroxyhispanone
Catalog No.:BCN7416
CAS No.:596814-48-1
- Cephalexin hydrochloride
Catalog No.:BCC4095
CAS No.:59695-59-9
- Piperacillin Sodium
Catalog No.:BCC4704
CAS No.:59703-84-3
- Camostat Mesilate
Catalog No.:BCC4894
CAS No.:59721-29-8
- Citalopram hydrobromide
Catalog No.:BCC7063
CAS No.:59729-32-7
- beta-Amyrin palmitate
Catalog No.:BCN4099
CAS No.:5973-06-8
- 1beta,10beta-Epoxy-6beta-isobutyryloxy-9-oxofuranoeremophilane
Catalog No.:BCN7601
CAS No.:59742-11-9
- Cudraflavanone B
Catalog No.:BCN3446
CAS No.:597542-74-0
The Effect of Glycopyrrolate on Nocturnal Sialorrhea in Patients Using Clozapine: A Randomized, Crossover, Double-Blind, Placebo-Controlled Trial.[Pubmed:28129312]
J Clin Psychopharmacol. 2017 Apr;37(2):155-161.
BACKGROUND: Nocturnal sialorrhea is one of the most frequent adverse events in clozapine treatment. Symptomatic management of sialorrhea usually consists of off-label treatment with anticholinergic agents. The aim of the current study is to evaluate the efficacy and safety of Glycopyrrolate in patients using clozapine that experience sialorrhea. METHODS: In a double-blind randomized crossover trial, patients with nocturnal sialorrhea (n = 32) were randomized to treatment with Glycopyrrolate 1 mg or placebo. This double-blinded phase was followed by an optional open label extension phase with Glycopyrrolate 2 mg. Exposure periods consisted of 6 consecutive days and were separated with 1 washout week. The primary outcome was clinical improvement of nocturnal sialorrhea assessed by the Patient Global Impression of Improvement (PGI-I). RESULTS: The proportion of patients with a clinical improvement according to PGI-I did not significantly differ between 1 mg and placebo (18.8% vs 6.3%, P = 0.289); however, in patients using Glycopyrrolate 2 mg once daily versus placebo, it did (43.5% vs 6.3%, P = 0.039). Glycopyrrolate was not associated with severe adverse events or worsening of cognitive adverse events. CONCLUSIONS: Glycopyrrolate 1 mg was not superior to placebo, whereas 2 mg showed a significant clinical improvement of nocturnal sialorrhea compared with placebo. Glycopyrrolate seemed to be a tolerable anticholinergic agent in the treatment of clozapine-associated sialorrhea.
The Effect of Nebulized Glycopyrrolate on Posterior Drooling in Patients with Brain Injury: Two Cases of Different Brain Lesions.[Pubmed:28081026]
Am J Phys Med Rehabil. 2017 Aug;96(8):e155-e158.
Posterior drooling, which can lead to substantial respiratory morbidity, including unexplained lung diseases and recurrent pneumonia, is an important issue in the rehabilitation unit. There are various treatment options for posterior drooling, including pharmacologic therapy, oral motor or behavioral therapy, biofeedback, local glandular injection of botulinum toxin, irradiation, and surgery. Among them, nebulized Glycopyrrolate has the following advantages: It is noninvasive and is relatively free of central adverse effects because it does not cross the blood-brain barrier unlike other anticholinergics. Although there has been one case report regarding the effectiveness of nebulized Glycopyrrolate for drooling in a motor neuron patient, there have not been any reports on its effectiveness for posterior drooling. Herein, we report two cases (an 82-year-old male bilateral hemiplegic stroke patient and a 1-year-old female cerebral palsy infant with bilaterally spastic hemiplegia of posterior drooling treated with nebulized Glycopyrrolate) and identify salivary aspiration and the effect of nebulized Glycopyrrolate using radionuclide salivagram. Considering its advantages and effectiveness, nebulized Glycopyrrolate should be considered as one of the reliable methods to manage posterior drooling in patients with impaired cognition or swallowing difficulties, such as severe brain injury.
Glycopyrrolate: It's time to review.[Pubmed:28183573]
J Clin Anesth. 2017 Feb;36:51-53.
Medication shortages have become an all-too-common inconvenience that has forced anesthesia providers to examine our administering practices. Because of these shortages, commonly used medications are at the greatest risk. Glycopyrrolate (Robinul), which has been in short supply in recent years, is one of the most widely used anticholinergic agents, especially in conjunction with the anticholinesterase neostigmine, for reversal of neuromuscular blockade (NMB) drugs. Here we review multiple studies from 1972 through 1986 that used varying methods of patient selection and dosage and drug combination criteria, and which noted that Glycopyrrolate had a superior efficacy and adverse effect profile when compared with atropine in NMB reversal. Meta-analysis from these studies indicated that the dosage of 0.2 mg of Glycopyrrolate for every 1 mg of neostigmine, given concomitantly (maximum 1 mg Glycopyrrolate and 5 mg neostigmine), demonstrated the greatest efficacy with the lowest incidence of unwanted adverse effects. It has now become common practice to use a dosage ratio of 0.2 mg Glycopyrrolate to 1.0 mg neostigmine for NMB reversal. Yet since 1986, there have been no studies on reversal with Glycopyrrolate and neostigmine. Frequent medication shortages and good medical practice should be an impetus for clinicians to reevaluate dosing practices of critical medications and revisit these drugs, such as Glycopyrrolate, with more current studies.


