Alpha-ObscurineCAS# 596-55-4 |
Quality Control & MSDS
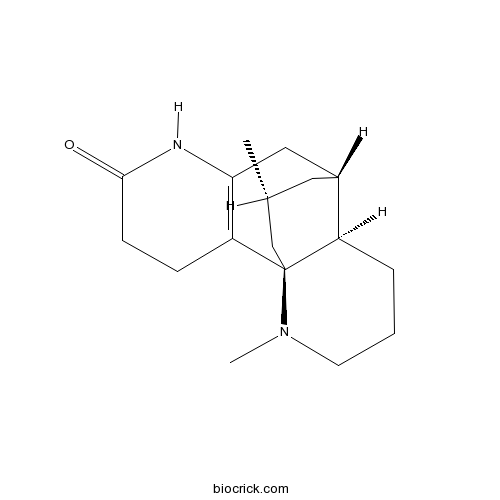
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 596-55-4 | SDF | Download SDF |
| PubChem ID | 5462446 | Appearance | Powder |
| Formula | C17H26N2O | M.Wt | 274.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,9S,10R,16R)-14,16-dimethyl-6,14-diazatetracyclo[7.5.3.01,10.02,7]heptadec-2(7)-en-5-one | ||
| SMILES | CC1CC2CC3=C(CCC(=O)N3)C4(C1)C2CCCN4C | ||
| Standard InChIKey | HXJHQEWSHQXRPH-IPJQOSJUSA-N | ||
| Standard InChI | InChI=1S/C17H26N2O/c1-11-8-12-9-15-14(5-6-16(20)18-15)17(10-11)13(12)4-3-7-19(17)2/h11-13H,3-10H2,1-2H3,(H,18,20)/t11-,12+,13-,17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | Chinese Traditional & Herbal Drugs,2015,46(1):33-7.Chemical constituents from whole herb of Lycopodium japonicum[Reference: WebLink]To study the chemical constituents in the whole herb of Lycopodium japonicum. |

Alpha-Obscurine Dilution Calculator

Alpha-Obscurine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6443 mL | 18.2216 mL | 36.4431 mL | 72.8863 mL | 91.1079 mL |
| 5 mM | 0.7289 mL | 3.6443 mL | 7.2886 mL | 14.5773 mL | 18.2216 mL |
| 10 mM | 0.3644 mL | 1.8222 mL | 3.6443 mL | 7.2886 mL | 9.1108 mL |
| 50 mM | 0.0729 mL | 0.3644 mL | 0.7289 mL | 1.4577 mL | 1.8222 mL |
| 100 mM | 0.0364 mL | 0.1822 mL | 0.3644 mL | 0.7289 mL | 0.9111 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Glycopyrrolate
Catalog No.:BCC4275
CAS No.:596-51-0
- H-D-Gln-OH
Catalog No.:BCC2920
CAS No.:5959-95-5
- 3-Amino-2-naphthoic acid
Catalog No.:BCC8607
CAS No.:5959-52-4
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- H-D-Ala-OtBu.HCl
Catalog No.:BCC2849
CAS No.:59531-86-1
- Boc-Ser-OBzl
Catalog No.:BCC3440
CAS No.:59524-02-6
- Megestrol Acetate
Catalog No.:BCC4365
CAS No.:595-33-5
- Soyasapogenol B
Catalog No.:BCN4097
CAS No.:595-15-3
- Calycanthine
Catalog No.:BCN7823
CAS No.:595-05-1
- Testosterone undecanoate
Catalog No.:BCC9173
CAS No.:5949-44-0
- Citric acid monohydrate
Catalog No.:BCN8492
CAS No.:5949-29-1
- Z-D-Glu(OBzl)-OH
Catalog No.:BCC2772
CAS No.:59486-73-6
- AC 55649
Catalog No.:BCC7359
CAS No.:59662-49-6
- Calyciphylline A
Catalog No.:BCN4098
CAS No.:596799-30-3
- 3-O-Acetyl-beta-boswellic acid
Catalog No.:BCN2672
CAS No.:5968-70-7
- 6alpha-Hydroxyhispanone
Catalog No.:BCN7416
CAS No.:596814-48-1
- Cephalexin hydrochloride
Catalog No.:BCC4095
CAS No.:59695-59-9
- Piperacillin Sodium
Catalog No.:BCC4704
CAS No.:59703-84-3
- Camostat Mesilate
Catalog No.:BCC4894
CAS No.:59721-29-8
- Citalopram hydrobromide
Catalog No.:BCC7063
CAS No.:59729-32-7
- beta-Amyrin palmitate
Catalog No.:BCN4099
CAS No.:5973-06-8
- 1beta,10beta-Epoxy-6beta-isobutyryloxy-9-oxofuranoeremophilane
Catalog No.:BCN7601
CAS No.:59742-11-9
- Cudraflavanone B
Catalog No.:BCN3446
CAS No.:597542-74-0
- Boc-Asp(OMe)-OH.DCHA
Catalog No.:BCC3367
CAS No.:59768-74-0
Lycopodium Alkaloids: Lycoplatyrine A, an Unusual Lycodine-Piperidine Adduct from Lycopodium platyrhizoma and the Absolute Configurations of Lycoplanine D and Lycogladine H.[Pubmed:30698428]
J Nat Prod. 2019 Feb 22;82(2):324-329.
Three new Lycopodium alkaloids comprising two lycodine-type alkaloids (1, 2) and one fawcettimine alkaloid (3), in addition to 16 known alkaloids, were isolated from Lycopodium platyrhizoma. The structures of these alkaloids were elucidated based on analysis of their NMR and MS data. Lycoplatyrine A (1) represents an unusual lycodine-piperidine adduct. The structures and absolute configurations of lycoplanine D (hydroxy-des- N-methyl-Alpha-Obscurine, 10) and lycogladine H (11) were confirmed by X-ray diffraction analysis.
Lycodine-Type Lycopodium Alkaloids from the Whole Plants of Huperzia serrata.[Pubmed:28744720]
Nat Prod Bioprospect. 2017 Oct;7(5):405-411.
Three new lycodine-type Lycopodium alkaloids, namely 1-methyllycodine (1), 8alpha-hydroxy-15,16-dehydro-des-N-methyl-Alpha-Obscurine (2), N-methyl-16-hydroxyhuperzine B (3), and one new natural lycodine-type Lycopodium alkaloid, N-methylhuperzine A (4), along with 11 known analogues (5-15), were isolated from the whole plants of club moss Huperzia serrata. The structures of 1-4 were elucidated on the basis of NMR spectroscopic and mass spectrometry data. Among them, compound 1 was the first lycodine-type alkaloid possessing a methyl group at C-1. In addition, the structure of 5 was confirmed by the single-crystal X-ray crystallography data and its (13)C NMR was reported for the first time in current study. Compounds 1-5 were tested their BACE1 inhibitory activity.
Isolation of a new lycodine alkaloid from Lycopodium japonicum.[Pubmed:25421949]
Nat Prod Res. 2015;29(8):735-8.
A new lycodine alkaloid, N-methylhydroxypropyllycodine (1), was isolated from the club moss Lycopodium japonicum Thunb, together with five known compounds, N-methyllycodine (2), huperzinine (3), beta-obscurine (4), Alpha-Obscurine (5) and des-N-methyl-Alpha-Obscurine (6). Their structures were elucidated by spectroscopic analyses, including 2D NMR techniques.
[Study on chemical constituents of Lycopodium alkaloids].[Pubmed:22667147]
Zhongguo Zhong Yao Za Zhi. 2012 Feb;37(4):475-7.
OBJECTIVE: To study the alkaloid chemical constituents of Lycopodium japonicum. METHOD: Compounds were isolated and purified by such methods as silica gel column chromatography, RP-C18 reversed phase column chromatography, Sephadex LH-20 column chromatography and Waters semi-preparative liquid chromatogram, and their structures were identified based on physicochemical property and spectrum data. RESULT: Nine known alkaloid chemical constituents were isolated and identified, they were lycodoline (1), lucidioline (2), Alpha-Obscurine (3), lycopodine (4), lycoposerramine-L (5), lycoposerramine-M (6), 11alpha-O-acetyl-lycopodine (7), des-N-methyl-a-obscurine (8), clavolonine (9). CONCLUSION: Compounds 4-9 were obtained from L. japonicum for the first time.


