Calyciphylline ACAS# 596799-30-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 596799-30-3 | SDF | Download SDF |
| PubChem ID | 10861970 | Appearance | Powder |
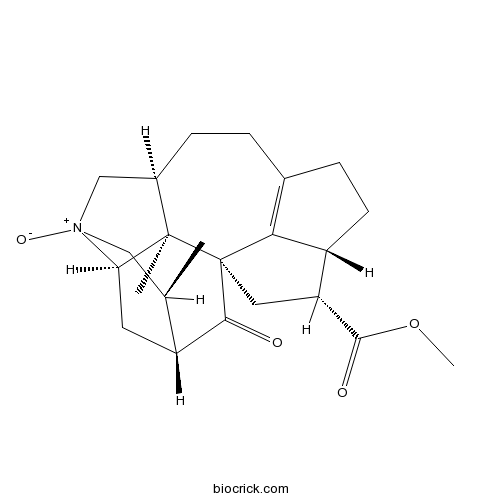
| Formula | C23H31NO4 | M.Wt | 385.5 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (1R,2S,3R,5R,6S,10S,16R,17R)-2,6-dimethyl-8-oxido-20-oxo-8-azoniahexacyclo[11.5.1.11,5.02,10.03,8.016,19]icos-13(19)-ene-17-carboxylate | ||
| SMILES | CC1C[N+]2(CC3CCC4=C5C(CC4)C(CC56C3(C2CC1C6=O)C)C(=O)OC)[O-] | ||
| Standard InChIKey | DLTJWHRTAHFESH-IGJFRSLJSA-N | ||
| Standard InChI | InChI=1S/C23H31NO4/c1-12-10-24(27)11-14-6-4-13-5-7-15-17(21(26)28-3)9-23(19(13)15)20(25)16(12)8-18(24)22(14,23)2/h12,14-18H,4-11H2,1-3H3/t12-,14-,15-,16-,17-,18-,22-,23+,24?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | Chem Asian J. 2015 Apr;10(4):865-8.Expedient construction of the [5-6-7] tricyclic core of calyciphylline a-type alkaloids.[Pubmed: 25377776]
Tetrahedron Lett. 2015 Jun 3;56(23):3503-3506.Toward the ABCD Core of the Calyciphylline A-Type Daphniphyllum Alkaloids: Solvent non-Innocence in Neutral Aminyl Radical Cyclizations.[Pubmed: 26028785]The Daphniphyllum alkaloids remain an attractive target in the synthetic community because of their unique framework and promising biological activities.
Org Lett. 2014 Feb 21;16(4):1072-5.Rapid access to the heterocyclic core of the calyciphylline A and daphnicyclidin A-type Daphniphyllum alkaloids via tandem cyclization of a neutral aminyl radical.[Pubmed: 24506430]A streamlined approach to the tertiary amine-containing core of the Calyciphylline A and daphnicyclidin A-type Daphniphyllum alkaloids is presented.
|

Calyciphylline A Dilution Calculator

Calyciphylline A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.594 mL | 12.9702 mL | 25.9403 mL | 51.8807 mL | 64.8508 mL |
| 5 mM | 0.5188 mL | 2.594 mL | 5.1881 mL | 10.3761 mL | 12.9702 mL |
| 10 mM | 0.2594 mL | 1.297 mL | 2.594 mL | 5.1881 mL | 6.4851 mL |
| 50 mM | 0.0519 mL | 0.2594 mL | 0.5188 mL | 1.0376 mL | 1.297 mL |
| 100 mM | 0.0259 mL | 0.1297 mL | 0.2594 mL | 0.5188 mL | 0.6485 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AC 55649
Catalog No.:BCC7359
CAS No.:59662-49-6
- Alpha-Obscurine
Catalog No.:BCN6701
CAS No.:596-55-4
- Glycopyrrolate
Catalog No.:BCC4275
CAS No.:596-51-0
- H-D-Gln-OH
Catalog No.:BCC2920
CAS No.:5959-95-5
- 3-Amino-2-naphthoic acid
Catalog No.:BCC8607
CAS No.:5959-52-4
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- H-D-Ala-OtBu.HCl
Catalog No.:BCC2849
CAS No.:59531-86-1
- Boc-Ser-OBzl
Catalog No.:BCC3440
CAS No.:59524-02-6
- Megestrol Acetate
Catalog No.:BCC4365
CAS No.:595-33-5
- Soyasapogenol B
Catalog No.:BCN4097
CAS No.:595-15-3
- Calycanthine
Catalog No.:BCN7823
CAS No.:595-05-1
- Testosterone undecanoate
Catalog No.:BCC9173
CAS No.:5949-44-0
- 3-O-Acetyl-beta-boswellic acid
Catalog No.:BCN2672
CAS No.:5968-70-7
- 6alpha-Hydroxyhispanone
Catalog No.:BCN7416
CAS No.:596814-48-1
- Cephalexin hydrochloride
Catalog No.:BCC4095
CAS No.:59695-59-9
- Piperacillin Sodium
Catalog No.:BCC4704
CAS No.:59703-84-3
- Camostat Mesilate
Catalog No.:BCC4894
CAS No.:59721-29-8
- Citalopram hydrobromide
Catalog No.:BCC7063
CAS No.:59729-32-7
- beta-Amyrin palmitate
Catalog No.:BCN4099
CAS No.:5973-06-8
- 1beta,10beta-Epoxy-6beta-isobutyryloxy-9-oxofuranoeremophilane
Catalog No.:BCN7601
CAS No.:59742-11-9
- Cudraflavanone B
Catalog No.:BCN3446
CAS No.:597542-74-0
- Boc-Asp(OMe)-OH.DCHA
Catalog No.:BCC3367
CAS No.:59768-74-0
- 6beta-Angeloyloxy-1beta,10beta-epoxy-9-oxofuranoeremophilane
Catalog No.:BCN7600
CAS No.:59780-08-4
- Cyclosporin C
Catalog No.:BCC8160
CAS No.:59787-61-0
Rapid access to the heterocyclic core of the calyciphylline A and daphnicyclidin A-type Daphniphyllum alkaloids via tandem cyclization of a neutral aminyl radical.[Pubmed:24506430]
Org Lett. 2014 Feb 21;16(4):1072-5.
A streamlined approach to the tertiary amine-containing core of the Calyciphylline A and daphnicyclidin A-type Daphniphyllum alkaloids is presented. A known carvone derivative is converted into the core structure in only four synthetic operations, and it is well poised for further elaboration. The key enabling methodology is a radical cyclization cascade beginning with addition of a secondary, neutral aminyl radical to the beta-position of an enone, followed by trapping with a pendant alkyne.
Expedient construction of the [5-6-7] tricyclic core of calyciphylline a-type alkaloids.[Pubmed:25377776]
Chem Asian J. 2015 Apr;10(4):865-8.
An efficient synthetic route toward the highly congested [5-6-7] tricyclic core of Calyciphylline A-type alkaloids has been developed. This approach features a highly efficient intramolecular Diels-Alder cycloaddition to establish the aza-five-membered C ring as well as the C1 all-carbon quaternary center, and a subsequent cyclopropanation together with a ring-expansion reaction of the resulted adduct to construct the seven-membered D ring.
Toward the ABCD Core of the Calyciphylline A-Type Daphniphyllum Alkaloids: Solvent non-Innocence in Neutral Aminyl Radical Cyclizations.[Pubmed:26028785]
Tetrahedron Lett. 2015 Jun 3;56(23):3503-3506.
The Daphniphyllum alkaloids remain an attractive target in the synthetic community because of their unique framework and promising biological activities. We have shown that the ABC core of the Calyciphylline A-type alkaloids can be rapidly accessed via the tandem cyclization of a neutral aminyl radical with a polarized cyclic olefin. Deuterium labeling experiments and reactions omitting a tin hydride reagent suggest that the solvent is the major source of the terminating hydrogen atom in the cyclization cascade. Incorporation of an internal alkyne in the radical pathway was tolerated in the reaction, and it provided the necessary atoms to enable completion of the D ring of the Calyciphylline A-type alkaloids.


