GSK-3 Inhibitor IX (BIO)GSK-3α/GSK-3β inhibitor, cell-permeable, ATP-competitive and reversible CAS# 667463-62-9 |
Quality Control & MSDS
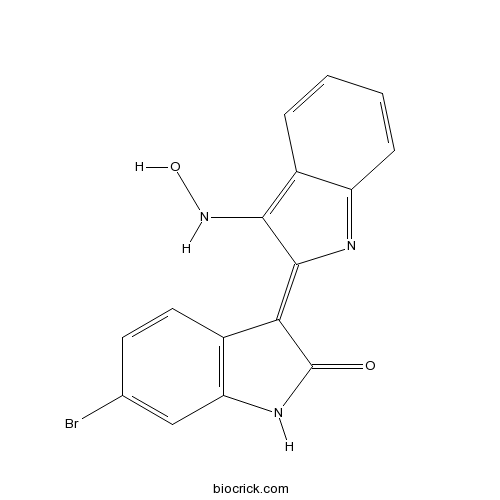
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 667463-62-9 | SDF | Download SDF |
| PubChem ID | 5287844 | Appearance | Powder |
| Formula | C16H10BrN3O2 | M.Wt | 356.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 6BIO | ||
| Solubility | DMSO : ≥ 23 mg/mL (64.58 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (3Z)-6-bromo-3-[3-(hydroxyamino)indol-2-ylidene]-1H-indol-2-one | ||
| SMILES | C1=CC2=C(C(=C3C4=C(C=C(C=C4)Br)NC3=O)N=C2C=C1)NO | ||
| Standard InChIKey | WNWSUJQVZJJGLF-SQFISAMPSA-N | ||
| Standard InChI | InChI=1S/C16H10BrN3O2/c17-8-5-6-9-12(7-8)19-16(21)13(9)15-14(20-22)10-3-1-2-4-11(10)18-15/h1-7,20,22H,(H,19,21)/b15-13- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective, ATP-competitive glycogen synthase kinase-3 (GSK-3) inhibitor (IC50 values are 5, 83, 300, 320 and > 10 000 nM at GSK-3, CDK5/p25, CDK2/cyclin A, CDK1/cyclin B and other common kinases respectively). Maintains self-renewal and pluripotency in embryonic stem cells via activation of the Wnt signaling pathway in vitro; also promotes proliferation and dedifferentiation in cardiomyocytes. Negative control available. |

GSK-3 Inhibitor IX (BIO) Dilution Calculator

GSK-3 Inhibitor IX (BIO) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8076 mL | 14.0382 mL | 28.0765 mL | 56.153 mL | 70.1912 mL |
| 5 mM | 0.5615 mL | 2.8076 mL | 5.6153 mL | 11.2306 mL | 14.0382 mL |
| 10 mM | 0.2808 mL | 1.4038 mL | 2.8076 mL | 5.6153 mL | 7.0191 mL |
| 50 mM | 0.0562 mL | 0.2808 mL | 0.5615 mL | 1.1231 mL | 1.4038 mL |
| 100 mM | 0.0281 mL | 0.1404 mL | 0.2808 mL | 0.5615 mL | 0.7019 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK-3 inhibitor IX (BIO) is a potent, selective, cell-permeable, ATP-competitive and reversible inhibitor of GSK-3α (glycogen synthase kinase-3α) and GSK-3β (lycogen synthase kinase-3β) (IC50 = 5nM for GSK-3β). [1]
GSK3 (glycogen synthase kinase-3) is a serine/threonine protein kinase which contributes to cell survival, diabetes, insulin resistance and Alzheimer's diseases. It is contributed to β-catenin/Wnt signaling pathway.
BIO facilitated the proliferation in mammalian cardiomyocytes by increasing the proliferation potential of cardiomyocytes. It induced S phase entry and beta-catenin activity in neonatal cardiomyocyte. [3] BIO also activated Wnt signaling and involved in maintaining pluripotency in human and mouse ESCs (embryonic stem cells). [2] In Cos-1 cells treated with 5 μm BIO for 24 hours, phosphorylation of β-catenin was inhibited on GSK-3 specific sites. In cell line deficient for AhR or ARNT, 10 μm BIO treatment for 24 hours showed its effect is through a direct and AhR- independent pathway.[1]
In vivo study showed BIO activated maternal Wnt signaling pathway in Xenopus embryos. It caused a hyper dorso-anteriorization at the expense of trunk and tail in a dose-response manner. It also activated the dorsal genes (siamois and chordin) ectopically. [1]
References:
[1] Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, Crovace C, Tarricone C, Musacchio A, Roe SM, Pearl L, Greengard P. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003 Dec;10(12):1255-66.
[2] Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004 Jan;10(1):55-63.
[3] Tseng AS, Engel FB, Keating MT. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem Biol. 2006 Sep;13(9):957-63.
- PG 931
Catalog No.:BCC6363
CAS No.:667430-81-1
- 5-Hydroxy-7-acetoxyflavone
Catalog No.:BCN4217
CAS No.:6674-40-4
- DBU
Catalog No.:BCC2840
CAS No.:6674-22-2
- 3-Nitro-L-tyrosine ethyl ester hydrochloride
Catalog No.:BCN1384
CAS No.:66737-54-0
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
- 8-Bromo-7-(but-2-yn-1-yl)-3-methyl-1H-purine-2,6(3H,7H)-dione
Catalog No.:BCC8786
CAS No.:666816-98-4
- Polygalacin D
Catalog No.:BCN3861
CAS No.:66663-91-0
- Platycodin D2
Catalog No.:BCN3847
CAS No.:66663-90-9
- Esculentoside H
Catalog No.:BCN2795
CAS No.:66656-92-6
- 7-Hydroxyflavone
Catalog No.:BCN3673
CAS No.:6665-86-7
- 5,7-Diacetoxyflavone
Catalog No.:BCN4216
CAS No.:6665-78-7
- Galangin 3-methyl ether
Catalog No.:BCN3681
CAS No.:6665-74-3
- BIO-acetoxime
Catalog No.:BCC6076
CAS No.:667463-85-6
- MeBIO
Catalog No.:BCC3898
CAS No.:667463-95-8
- NSC 109555 ditosylate
Catalog No.:BCC7540
CAS No.:66748-43-4
- Diosbulbin D
Catalog No.:BCN4218
CAS No.:66756-57-8
- 6,8-Diprenylorobol
Catalog No.:BCN4602
CAS No.:66777-70-6
- Esculin Sesquihydrate
Catalog No.:BCC8324
CAS No.:66778-17-4
- Platycodin A
Catalog No.:BCN7997
CAS No.:66779-34-8
- Impurity B of Calcitriol
Catalog No.:BCC1645
CAS No.:66791-71-7
- Syringaresinol-di-O-glucoside
Catalog No.:BCN2600
CAS No.:66791-77-3
- (+/-)-Forbesione
Catalog No.:BCN6423
CAS No.:667914-50-3
- Hernandezine
Catalog No.:BCN7793
CAS No.:6681-13-6
- Jatrorrhizine chloride
Catalog No.:BCN4956
CAS No.:6681-15-8
The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes.[Pubmed:16984885]
Chem Biol. 2006 Sep;13(9):957-63.
The maintenance of self-renewal in stem cells appears to be distinct from the induction of proliferation of the terminally differentiated mammalian cardiomyocytes because it is believed that the latter are unable to divide. However, proliferation is a necessary step in both processes. Interestingly, the small molecule 6-bromoindirubin-3'-oxime (BIO) is the first pharmacological agent shown to maintain self-renewal in human and mouse embryonic stem cells. To determine whether a molecule that can maintain stem cell properties can also participate in controlling the proliferative capability of the highly differentiated cardiomyocytes, we examine the effect of BIO in postmitotic cardiac cells. Here, we show that BIO promotes proliferation in mammalian cardiomyocytes. Our demonstration of a second role for BIO suggests that the maintenance of stem cell self-renewal and the induction of proliferation in differentiated cardiomyocytes may share common molecular pathways.
Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases.[Pubmed:14761195]
J Med Chem. 2004 Feb 12;47(4):935-46.
Pharmacological inhibitors of glycogen synthase kinase-3 (GSK-3) and cyclin-dependent kinases have a promising potential for applications against several neurodegenerative diseases such as Alzheimer's disease. Indirubins, a family of bis-indoles isolated from various natural sources, are potent inhibitors of several kinases, including GSK-3. Using the cocrystal structures of various indirubins with GSK-3beta, CDK2 and CDK5/p25, we have modeled the binding of indirubins within the ATP-binding pocket of these kinases. This modeling approach provided some insight into the molecular basis of indirubins' action and selectivity and allowed us to forecast some improvements of this family of bis-indoles as kinase inhibitors. Predicted molecules, including 6-substituted and 5,6-disubstituted indirubins, were synthesized and evaluated as CDK and GSK-3 inhibitors. Control, kinase-inactive indirubins were obtained by introduction of a methyl substitution on N1.
GSK-3-selective inhibitors derived from Tyrian purple indirubins.[Pubmed:14700633]
Chem Biol. 2003 Dec;10(12):1255-66.
Gastropod mollusks have been used for over 2500 years to produce the "Tyrian purple" dye made famous by the Phoenicians. This dye is constituted of mixed bromine-substituted indigo and indirubin isomers. Among these, the new natural product 6-bromoindirubin and its synthetic, cell-permeable derivative, 6-bromoindirubin-3'-oxime (BIO), display remarkable selective inhibition of glycogen synthase kinase-3 (GSK-3). Cocrystal structure of GSK-3beta/BIO and CDK5/p25/indirubin-3'-oxime were resolved, providing a detailed view of indirubins' interactions within the ATP binding pocket of these kinases. BIO but not 1-methyl-BIO, its kinase inactive analog, also inhibited the phosphorylation on Tyr276/216, a GSK-3alpha/beta activation site. BIO but not 1-methyl-BIO reduced beta-catenin phosphorylation on a GSK-3-specific site in cellular models. BIO but not 1-methyl-BIO closely mimicked Wnt signaling in Xenopus embryos. 6-bromoindirubins thus provide a new scaffold for the development of selective and potent pharmacological inhibitors of GSK-3.


