GSK 137647FFA4/GPR120 agonist CAS# 349085-82-1 |
- Adefovir Dipivoxil
Catalog No.:BCC5025
CAS No.:142340-99-6
- Merimepodib
Catalog No.:BCC4128
CAS No.:198821-22-6
- Telbivudine
Catalog No.:BCC3862
CAS No.:3424-98-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 349085-82-1 | SDF | Download SDF |
| PubChem ID | 743974 | Appearance | Powder |
| Formula | C16H19NO3S | M.Wt | 305.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GSK 137647 | ||
| Solubility | DMSO : ≥ 100 mg/mL (327.45 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
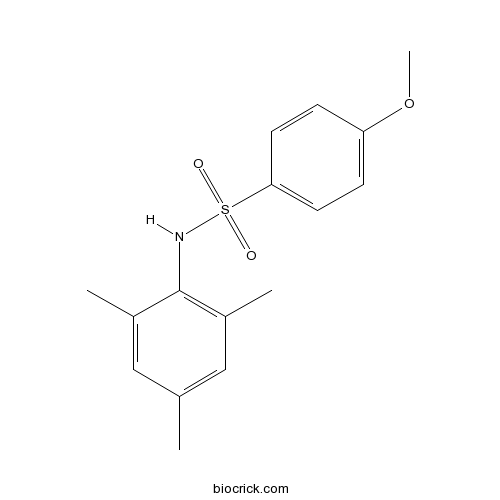
| Chemical Name | 4-methoxy-N-(2,4,6-trimethylphenyl)benzenesulfonamide | ||
| SMILES | CC1=CC(=C(C(=C1)C)NS(=O)(=O)C2=CC=C(C=C2)OC)C | ||
| Standard InChIKey | FQUAFMNPXPXOJE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective FFA4 (GPR120) agonist (pEC50 values are 6.3, 6.2 and 6.1 at the human, mouse and rat receptor, respectively). Exhibits ≥100-fold selectivity against a panel of 61 targets including FFA1, FFA2 and FFA3. Enhances glucose-stimulated insulin secretion in MIN6 cells. Induces intracellular calcium accumulation in U2OS cells; this activity is inhibited by AH 7614. | |||||

GSK 137647 Dilution Calculator

GSK 137647 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2745 mL | 16.3725 mL | 32.745 mL | 65.49 mL | 81.8625 mL |
| 5 mM | 0.6549 mL | 3.2745 mL | 6.549 mL | 13.098 mL | 16.3725 mL |
| 10 mM | 0.3275 mL | 1.6373 mL | 3.2745 mL | 6.549 mL | 8.1863 mL |
| 50 mM | 0.0655 mL | 0.3275 mL | 0.6549 mL | 1.3098 mL | 1.6373 mL |
| 100 mM | 0.0327 mL | 0.1637 mL | 0.3275 mL | 0.6549 mL | 0.8186 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Target: FFA4/GPR120
IC50: N/A
GSK137647A is potent and selective FFA4/GPR120 agonist with pEC50 values of 6.3, 6.2, and 6.1 at the human, mouse and rat receptor, respectively [1]. The free fatty acid receptor 4 (FFA4/GPR120), a member of the G protein-coupled receptor family, is a potential 7TM receptor involved in long-chain fatty acid-stimulated glucagon-like peptide-1 (GLP-1) secretion. FFA4 is highly expressed in the intestinal endocrine cell line STC-1 and the intestine [1]. GLP-1 regulates multiple physiological functions including eating behavior [2].
In vitro: GSK137647A (50 μM) induced a concentration-dependent increase in glucose (25 mM)-stimulated insulin secretion in MIN6 mouse insulinoma cell line [1]. In addition, GSK137647A (100 μM) induced a modest increase in GLP-1 secretion in the human intestinal cell line NCI-H716. Moreover, GSK137647A induced intracellular calcium accumulation in U2OS cells [1].
In vivo: GSK137647A (50 μM) induced active GLP-1 release by mouse circumvallate papillae (CVPs) [2].
References:
1. Sparks SM, Chen G, Collins JL, Danger D, Dock ST, Jayawickreme C, et al. Identification of diarylsulfonamides as agonists of the free fatty acid receptor 4 (FFA4/GPR120). Bioorg Med Chem Lett. 2014;24(14):3100-3.
2. Martin C, Passilly-Degrace P, Chevrot M, Ancel D, Sparks SM, Drucker DJ, et al. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. J Lipid Res. 2012;53(11):2256-65.
- HC-030031
Catalog No.:BCC4910
CAS No.:349085-38-7
- Vineridine
Catalog No.:BCN5286
CAS No.:3489-06-3
- UK 383367
Catalog No.:BCC2308
CAS No.:348622-88-8
- Palmatine
Catalog No.:BCN5285
CAS No.:3486-67-7
- Coptisine
Catalog No.:BCN6320
CAS No.:3486-66-6
- Optovin
Catalog No.:BCC6323
CAS No.:348575-88-2
- Methyl 3-methoxyacrylate
Catalog No.:BCN2258
CAS No.:34846-90-7
- 12-Hydroxyabietic acid
Catalog No.:BCN5284
CAS No.:3484-61-5
- Metiamide
Catalog No.:BCC1742
CAS No.:34839-70-8
- Bz-Tyr-Oet
Catalog No.:BCC3122
CAS No.:3483-82-7
- DL-Dithiothreitol
Catalog No.:BCC7586
CAS No.:3483-12-3
- 4'-Demethyleucomin
Catalog No.:BCN5283
CAS No.:34818-83-2
- JX 401
Catalog No.:BCC7443
CAS No.:349087-34-9
- Cryptomeridiol 11-rhamnoside
Catalog No.:BCN4648
CAS No.:349112-30-7
- S-Adenosyl-L-Methionine iodide salt
Catalog No.:BCN2232
CAS No.:3493-13-8
- Alda 1
Catalog No.:BCC6096
CAS No.:349438-38-6
- Methyl dodonate A acetate
Catalog No.:BCN4647
CAS No.:349487-98-5
- Methyl dodonate A
Catalog No.:BCN4646
CAS No.:349534-70-9
- Dodonolide
Catalog No.:BCN4645
CAS No.:349534-73-2
- 3'',4''-Di-O-acetyl-2'',6''-di-O-p-coumaroylastragalin
Catalog No.:BCN6843
CAS No.:349545-02-4
- Z-Val-OSu
Catalog No.:BCC2733
CAS No.:3496-11-5
- Kushenol F
Catalog No.:BCN3798
CAS No.:34981-24-3
- Kuraridine
Catalog No.:BCN2988
CAS No.:34981-25-4
- Kurarinone
Catalog No.:BCN2985
CAS No.:34981-26-5
Activation of Ras-ERK Signaling and GSK-3 by Amyloid Precursor Protein and Amyloid Beta Facilitates Neurodegeneration in Alzheimer's Disease.[Pubmed:28374012]
eNeuro. 2017 Mar 27;4(2). pii: eN-NWR-0149-16.
It is widely accepted that amyloid beta (Abeta) generated from amyloid precursor protein (APP) oligomerizes and fibrillizes to form neuritic plaques in Alzheimer's disease (AD), yet little is known about the contribution of APP to intracellular signaling events preceding AD pathogenesis. The data presented here demonstrate that APP expression and neuronal exposure to oligomeric Abeta42 enhance Ras/ERK signaling cascade and glycogen synthase kinase 3 (GSK-3) activation. We find that RNA interference (RNAi)-directed knockdown of APP in B103 rat neuroblastoma cells expressing APP inhibits Ras-ERK signaling and GSK-3 activation, indicating that APP acts upstream of these signal transduction events. Both ERK and GSK-3 are known to induce hyperphosphorylation of tau and APP at Thr668, and our findings suggest that aberrant signaling by APP facilitates these events. Supporting this notion, analysis of human AD brain samples showed increased expression of Ras, activation of GSK-3, and phosphorylation of APP and tau, which correlated with Abeta levels in the AD brains. Furthermore, treatment of primary rat neurons with Abeta recapitulated these events and showed enhanced Ras-ERK signaling, GSK-3 activation, upregulation of cyclin D1, and phosphorylation of APP and tau. The finding that Abeta induces Thr668 phosphorylation on APP, which enhances APP proteolysis and Abeta generation, denotes a vicious feedforward mechanism by which APP and Abeta promote tau hyperphosphorylation and neurodegeneration in AD. Based on these results, we hypothesize that aberrant proliferative signaling by APP plays a fundamental role in AD neurodegeneration and that inhibition of this would impede cell cycle deregulation and neurodegeneration observed in AD.
Transient Cerebral Ischemia Alters GSK-3beta and p-GSK-3beta Immunoreactivity in Pyramidal Neurons and Induces p-GSK-3beta Expression in Astrocytes in the Gerbil Hippocampal CA1 Area.[Pubmed:28349361]
Neurochem Res. 2017 Aug;42(8):2305-2313.
Glycogen synthase kinase 3beta (GSK-3beta) is a key downstream protein in the PI3K/Akt pathway. Phosphorylation of serine 9 of GSK-3beta (GSK-3beta activity inhibition) promotes cell survival. In this study, we examined changes in expressions of GSK-3beta and phosphorylation of GSK-3beta (p-GSK-3beta) in the gerbil hippocampal CA1 area after 5 min of transient cerebral ischemia. GSK-3beta immunoreactivity in the CA1 area was increased in pyramidal cells at 6 h after ischemia-reperfusion. It was decreased in CA1 pyramidal cells from 12 h after ischemia-reperfusion, and hardly detected in the CA1 pyramidal cells at 5 days after ischemia-reperfusion. p-GSK-3beta immunoreactivity was slightly decreased in CA1 pyramidal cells at 6 and 12 h after ischemia-reperfusion. It was significantly increased in these cells at 1 and 2 days after ischemia-reperfusion. Five days after ischemia-reperfusion, p-GSK-3beta immunoreactivity was hardly found in CA1 pyramidal cells. However, p-GSK-3beta immunoreactivity was strongly expressed in astrocytes primarily distributed in strata oriens and radiatum. In conclusion, GSK-3beta and p-GSK-3beta were significantly changed in pyramidal cells and/or astrocytes in the gerbil hippocampal CA1 area following 5 min of transient cerebral ischemia. This finding indicates that GSK-3beta and p-GSK-3beta are closely related to delayed neuronal death.
GSK-3-mediated phosphorylation couples ER-Golgi transport and nuclear stabilization of the CREB-H transcription factor to mediate apolipoprotein secretion.[Pubmed:28381424]
Mol Biol Cell. 2017 Jun 1;28(11):1565-1579.
CREB-H, an ER-anchored transcription factor, plays a key role in regulating secretion in metabolic pathways, particularly triglyceride homeostasis. It controls the production both of secretory pathway components and cargoes, including apolipoproteins ApoA-IV and ApoC-II, contributing to VLDL/HDL distribution and lipolysis. The key mechanism controlling CREB-H activity involves its ER retention and forward transport to the Golgi, where it is cleaved by Golgi-resident proteases, releasing the N-terminal product, which traffics to the nucleus to effect transcriptional responses. Here we show that a serine-rich motif termed the P-motif, located in the N-terminus between serines 73 and 90, controls release of the precursor transmembrane form from the ER and its forward transport to the Golgi. This motif is subject to GSK-3 phosphorylation, promoting ER retention, while mutation of target serines and drug inhibition of GSK-3 activity coordinately induce both forward transport of the precursor and cleavage, resulting in nuclear import. We previously showed that for the nuclear product, the P-motif is subject to multiple phosphorylations, which regulate stability by targeting the protein to the SCF(Fbw1a) E3 ubiquitin ligase. Thus phosphorylation at the P-motif provides integrated control of CREB-H function, coupling intercompartmental transport in the cytoplasm with stabilization of the active form in the nucleus.
SLM, a novel carbazole-based fluorophore attenuates okadaic acid-induced tau hyperphosphorylation via down-regulating GSK-3beta activity in SH-SY5Y cells.[Pubmed:28359686]
Eur J Pharm Sci. 2017 Dec 15;110:101-108.
Phosphorylated tau dissociates from microtubules and aggregates to form neurofibrillary tangles resulting in neuronal toxicity and cognitive deficits. Attenuating tau hyperphosphorylation is considered as an effective therapeutic approach for Alzheimer's disease (AD). From our previous study, SLM, a carbazole-based fluorophore prevents Abeta aggregation, reduced glycogen synthase kinase-3beta (GSK-3beta) activity and tau hyperphosphorylation in triple transgenic mouse model of AD. However, the mechanism by which SLM attenuates tau hyperphosphorylation warrants further investigation. In the current study, we intend to evaluate the effects of SLM against okadaic acid (OA)-induced tau hyperphosphorylation and microtubules instability in human neuroblastoma (SH-SY5Y) cells. The results showed that, SLM reduced the OA-induced cell neurotoxicity and tau hyperphosphorylation in SH-SY5Y cells. SLM treatment down-regulated GSK-3beta activity. However, in the presence of GSK-3beta inhibitor (SB216763, 10muM), SLM treatment could not reduce GSK-3beta activity and tau hyperphosphorylation as compared with SB216763 treatment alone. Furthermore, SLM treatment also ameliorated OA-induced microtubules instability and cytoskeleton damage. Collectively, SLM attenuated OA-induced tau hyperphosphorylation via down-regulating GSK-3beta activity in SH-SY5Y cells. Therefore, this study supports SLM as a potential compound for AD and other tau pathology-related neurodegenerative disorders.
Identification of diarylsulfonamides as agonists of the free fatty acid receptor 4 (FFA4/GPR120).[Pubmed:24881566]
Bioorg Med Chem Lett. 2014 Jul 15;24(14):3100-3.
The exploration of a diarylsulfonamide series of free fatty acid receptor 4 (FFA4/GPR120) agonists is described. This work led to the identification of selective FFA4 agonist 8 (GSK137647A) and selective FFA4 antagonist 39. The in vitro profile of compounds 8 and 39 is presented herein.
Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity.[Pubmed:22904345]
J Lipid Res. 2012 Nov;53(11):2256-65.
Glucagon-like peptide-1 (GLP-1) signaling modulates sweet-taste sensitivity in the mouse. Because circumvallate papillae (CVPs) express both GLP-1 and its receptor, a local regulation has been suggested. However, whether dietary lipids are involved in this regulation, as shown in the gut, is unknown. By using a combination of biochemical, immunohistochemical, and behavioral approaches, the present data i) confirm the role of GLP-1 signaling in the attraction for sucrose, ii) demonstrate that minute quantities of long-chain FAs (LCFAs) reinforce the attraction for sucrose in a GLP-1 receptor-dependent manner, iii) suggest an involvement of the LCFA receptor GPR120 expressed in taste buds in this system, and iv) support the existence of a regulation by GLP-1 of the lipid sensing mediated by lingual CD36. Therefore, oro-sensory detection of LCFAs may affect sweet and fatty taste responsiveness by controlling the secretion of lingual GLP-1. This regulatory loop, probably triggered by the LCFA-GPR120 interaction, might contribute to the high palatability of foods rich both in fat and sugar.


