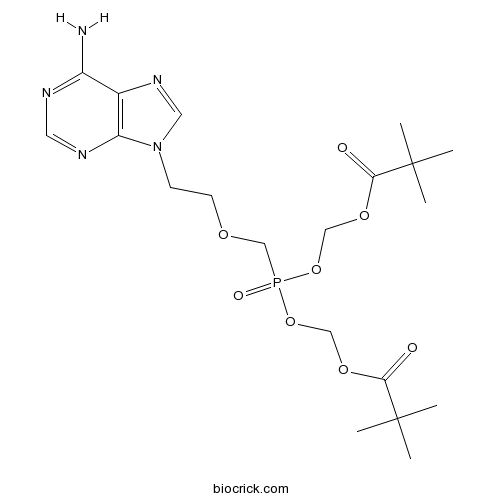
Adefovir DipivoxilCAS# 142340-99-6 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- DPH
Catalog No.:BCC1538
CAS No.:484049-04-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 142340-99-6 | SDF | Download SDF |
| PubChem ID | 60871 | Appearance | Powder |
| Formula | C20H32N5O8P | M.Wt | 501.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GS 0840 | ||
| Solubility | DMSO : ≥ 100 mg/mL (199.41 mM) H2O : 0.67 mg/mL (1.34 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [2-(6-aminopurin-9-yl)ethoxymethyl-(2,2-dimethylpropanoyloxymethoxy)phosphoryl]oxymethyl 2,2-dimethylpropanoate | ||
| SMILES | CC(C)(C)C(=O)OCOP(=O)(COCCN1C=NC2=C1N=CN=C2N)OCOC(=O)C(C)(C)C | ||
| Standard InChIKey | WOZSCQDILHKSGG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H32N5O8P/c1-19(2,3)17(26)30-11-32-34(28,33-12-31-18(27)20(4,5)6)13-29-8-7-25-10-24-14-15(21)22-9-23-16(14)25/h9-10H,7-8,11-13H2,1-6H3,(H2,21,22,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Adefovir Dipivoxil works by blocking reverse transcriptase, an enzyme that is crucial for the hepatitis B virus (HBV) to reproduce in the body.
Target: NRTIs; HBV
Adefovir Dipivoxil works by blocking reverse transcriptase, an enzyme that is crucial for the hepatitis B virus (HBV) to reproduce in the body. Adefovir Dipivoxil is used for treatment of hepatitis B and herpes simplex virus infection [1-3]. Adefovir Dipivoxil is approved for the treatment of chronic hepatitis B in adults with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (primarily ALT) or histologically active disease. Adefovir Dipivoxil is a failed treatment for HIV[3, 4]. References: | |||||

Adefovir Dipivoxil Dilution Calculator

Adefovir Dipivoxil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9941 mL | 9.9707 mL | 19.9414 mL | 39.8827 mL | 49.8534 mL |
| 5 mM | 0.3988 mL | 1.9941 mL | 3.9883 mL | 7.9765 mL | 9.9707 mL |
| 10 mM | 0.1994 mL | 0.9971 mL | 1.9941 mL | 3.9883 mL | 4.9853 mL |
| 50 mM | 0.0399 mL | 0.1994 mL | 0.3988 mL | 0.7977 mL | 0.9971 mL |
| 100 mM | 0.0199 mL | 0.0997 mL | 0.1994 mL | 0.3988 mL | 0.4985 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Adefovir Dipivoxil works by blocking reverse transcriptase, an enzyme that is crucial for the hepatitis B virus (HBV) to reproduce in the body.
- L-701,324
Catalog No.:BCC6842
CAS No.:142326-59-8
- Teuvincenone H
Catalog No.:BCN6227
CAS No.:142299-73-8
- Shizukaol D
Catalog No.:BCN6226
CAS No.:142279-42-3
- Shizukaol C
Catalog No.:BCN6225
CAS No.:142279-41-2
- Shizukaol B
Catalog No.:BCN6983
CAS No.:142279-40-1
- Kenpaullone
Catalog No.:BCC7047
CAS No.:142273-20-9
- Rauvotetraphylline E
Catalog No.:BCN7051
CAS No.:1422506-53-3
- Rauvotetraphylline D
Catalog No.:BCN7054
CAS No.:1422506-52-2
- Rauvotetraphylline C
Catalog No.:BCN7055
CAS No.:1422506-51-1
- Rauvotetraphylline B
Catalog No.:BCN7056
CAS No.:1422506-50-0
- Rauvotetraphylline A
Catalog No.:BCN7052
CAS No.:1422506-49-7
- 2'-Rhamnoechinacoside
Catalog No.:BCN8219
CAS No.:1422390-59-7
- H-D-Phe-pNA
Catalog No.:BCC3015
CAS No.:14235-18-8
- SP2509
Catalog No.:BCC5578
CAS No.:1423715-09-6
- FR 139317
Catalog No.:BCC5733
CAS No.:142375-60-8
- 7alpha-Hydroxy-4,11-cadinadiene-3,8-dione
Catalog No.:BCN7057
CAS No.:1423809-64-6
- Didemethylpseudoaspidin AA
Catalog No.:BCN3777
CAS No.:142382-28-3
- Mesterolone
Catalog No.:BCC9023
CAS No.:1424-00-6
- Crovatin
Catalog No.:BCN2517
CAS No.:142409-09-4
- NHS-SS-Biotin
Catalog No.:BCC3581
CAS No.:142439-92-7
- Myricetin 3-O-beta-D-xylopyranosyl(1-2)-beta-D-glucopyranoside
Catalog No.:BCN8140
CAS No.:142449-93-2
- Lobetyolinin
Catalog No.:BCN3322
CAS No.:142451-48-7
- Amthamine dihydrobromide
Catalog No.:BCC6744
CAS No.:142457-00-9
- 3-O-beta-D-apiofuranosyl(1-2)-beta-D-glucopyranosyl rhamnocitrin 4-O-beta-D-glucopyranoside
Catalog No.:BCN8141
CAS No.:142473-99-2
Osteomalacia induced by long-term low-dose adefovir dipivoxil: Clinical characteristics and genetic predictors.[Pubmed:27664568]
Bone. 2016 Dec;93:97-103.
CONTEXT: Adefovir Dipivoxil (ADV) was an important cause of adult-onset hypophosphatemic osteomalacia. However, its clinical characteristics and mechanisms have not been well defined. OBJECTIVE: The objective of the study was to summarize the clinical characteristics of ADV-induced osteomalacia and to explore the association between ADV-associated tubulopathy and polymorphisms in genes encoding drug transporters. DESIGN, SETTING, PATIENTS, AND MAIN OUTCOME MEASURE: Seventy-six affected patients were clinically studied. The SLC22A6 and ABCC2 genes were screened and compared with healthy people from the HapMap. RESULTS: Hypophosphatemia, high serum alkaline phosphatase (ALP) levels, hypouricemia, nondiabetic glycosuria, proteinuria, metabolic acidosis and high bone turnover markers were the main metabolic characteristics. Fractures and pseudofractures occurred in 39 patients. Stopping ADV administration, supplementing calcitriol and calcium was effective during the follow-up period. Single SNP analysis revealed a higher percentage of the G/A genotype at c.2934 in exon 22 of the ABCC2 gene (rs3740070) in patients than in healthy people (12% [7 of 58 patients] vs. 0% [0 of 45 patients]; P=0.017), while there was no subject with homozygosity for the A allele at c.2934. CONCLUSIONS: ADV can be nephrotoxic at a conventional dosage. The G/A genotype at c.2934 of the ABCC2 gene may be a predictor of patients at greater risk for developing ADV-associated tubulopathy. Larger case-control studies are needed to further verify this finding.
Relationship between nephrotoxicity and long-term adefovir dipivoxil therapy for chronic hepatitis B: A meta-analysis.[Pubmed:27977591]
Medicine (Baltimore). 2016 Dec;95(50):e5578.
BACKGROUND: To assess the relationship between Adefovir Dipivoxil and renal function after anti-hepatitis B virus therapy and elucidate the risk factors involved. METHODS: Based on the requirements of the Cochrane systematic review methodology, 21 observational articles on Adefovir Dipivoxil-associated renal dysfunction were obtained by searching various databases, between January 1, 1995 and July 1, 2016. The Newcastle Ottawa Scale was used to evaluate risk bias. Parameters for 4276 chronic hepatitis B patients were analyzed by Review Manager and R software, and glomerular filtration rate, creatinine clearance, and serum creatinine values were extracted to evaluate renal function. RESULTS: Renal dysfunction was more likely to occur in patients receiving the Adefovir Dipivoxil therapy (odds ratio [OR] 1.98, 95% confidence interval [CI] 1.40-2.80) than the none-Adefovir Dipivoxil group. Subgroup analysis showed that renal function predictive value is higher for glomerular filtration rate (OR 2.42, 95% CI 1.34-3.14), compared with serum creatinine levels (OR 1.51, 95% CI 0.75-3.04). The rate of Adefovir Dipivoxil-associated renal dysfunction was 12% (95% CI 0.08-0.16). Older patients and patients with renal insufficiency, hypertension, and diabetes mellitus were more prone to developing Adefovir Dipivoxil-associated renal dysfunction; however, integrated raw data were insufficient for further detailed analysis. CONCLUSION: Long-term Adefovir Dipivoxil therapy is connected to renal dysfunction in chronic hepatitis B, necessitating the monitoring of kidney function.
Adefovir dipivoxil-induced Fanconi syndrome and its predictive factors: A study of 28 cases.[Pubmed:28123560]
Oncol Lett. 2017 Jan;13(1):307-314.
The aim of the present study was to identify monitoring and prevention measures as well as predictive factors for early detection of renal toxicity associated with long-term administration of Adefovir Dipivoxil in order to avoid progression to Fanconi syndrome. Clinical data of 28 patients with Fanconi syndrome caused by long-term administration of Adefovir Dipivoxil for the treatment of chronic hepatitis B virus (HBV) infection were collected pre-and post-administration for analysis. Patients presented with fatigue, progressive systemic pain in multiple bones and joints, as well as difficulty in walking and pathological fractures in a number of severe cases. Laboratory examinations revealed hypophosphatemia, elevated serum cystatin C (Cys-C), elevated serum creatinine (SCr), reduced glomerular filtration rate (GFR), positive urinary protein, erythrocytes and glucose, as well as osteoporosis. In consequence, Adefovir Dipivoxil administration was stopped, and patients received concentrated divitamins, sodium phosphate syrup and calcitriol. Symptoms and abnormalities in laboratory examinations were significantly improved in all patients after 2-6 months. Therefore, serum phosphate, SCr, routine urine parameters, Cys-C and GFR should be monitored regularly in chronic HBV patients treated with Adefovir Dipivoxil. The following factors were identified as predictive of kidney damage and Fanconi syndrome: Age >/=40 years, living in rural areas, previous renal toxicity, estimated GFR (eGFR) <90 ml/min/1.73 m(2), hypertension, diabetes, cirrhosis and duration of Adefovir Dipivoxil treatment exceeding 24 months. The present results indicate that timely termination of Adefovir Dipivoxil treatment and replacement with other antiviral agents is critical once renal impairment appears, and that it is necessary to change to other antiviral agents and prolong the interval of administration according to the eGFR level.
Adefovir dipivoxil is less expensive than lamivudine and associated with similar prognosis in patients with hepatitis B virus-related hepatocellular carcinoma after radical resection.[Pubmed:27877054]
Onco Targets Ther. 2016 Nov 10;9:6897-6907.
AIM: Lamivudine (LAM) and Adefovir Dipivoxil (ADV) are widely used in patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC), but few studies have directly compared their therapeutic efficacy and treatment cost. This study aims to compare LAM with ADV head-to-head in these patients. METHODS: We retrospectively analyzed 201 patients with HBV-related HCC who underwent radical resection and subsequently received LAM (n=155) or ADV (n=46). The two groups were compared in terms of HBV-DNA levels, liver function, antiviral resistance, recurrence-free, and overall survival, as well as antiviral medication costs. RESULTS: Despite significant improvement in HBV-DNA and alanine aminotransferase level in the LAM group after 1 year of antiviral therapy, these parameters did not differ significantly between the two groups over the following 2 years. Incidence of antiviral resistance after 1, 2, and 3 years of antiviral treatment was significantly higher in the LAM group (19.5%, 45.7%, and 56.4%) than in the ADV group (0%, 3.3%, and 14.5%; P<0.001). Overall survival at 1, 2, and 3 years after resection was similar for the LAM group (84.5%, 69.3%, and 64.6%) and the ADV group (84.1%, 77.8%, and 63.4%; P=0.905). Recurrence-free survival at the three follow-up points was also similar for the LAM group (71.7%, 58.3%, and 43.9%) and the ADV group (81.1%, 66.1%, and 53.0%; P=0.452). Cox regression analysis confirmed that both nucleos(t)ide analogues were associated with similar overall and recurrence-free survival. However, the average medication costs after 1, 2, and 3 years of antiviral treatment were significantly higher in the LAM group (euro3.0, euro4.8, and euro5.6 per person per day) than in the ADV group (euro2.2, euro2.4, and euro3.1 per person per day; all P<0.05). CONCLUSION: ADV and LAM are associated with similar survival benefit in patients with HBV-related HCC after radical resection, but ADV is more cost-effective.


