Methyl 3-methoxyacrylateCAS# 34846-90-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 34846-90-7 | SDF | Download SDF |
| PubChem ID | 5323651 | Appearance | Oil |
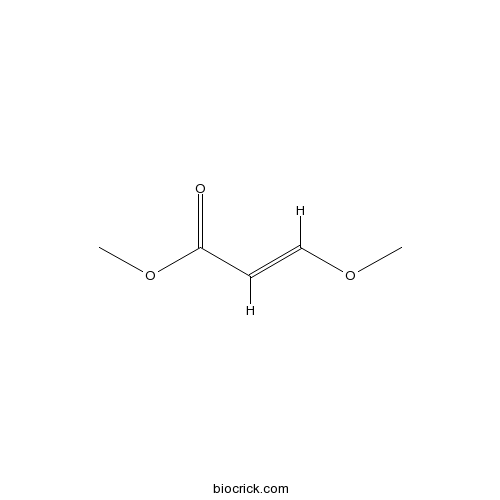
| Formula | C5H8O3 | M.Wt | 116.12 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (E)-3-methoxyprop-2-enoate | ||
| SMILES | COC=CC(=O)OC | ||
| Standard InChIKey | AUTCCPQKLPMHDN-ONEGZZNKSA-N | ||
| Standard InChI | InChI=1S/C5H8O3/c1-7-4-3-5(6)8-2/h3-4H,1-2H3/b4-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Organic Process Research & Development, 2002, 6(4):357-366.Process Research on the Synthesis of Silthiofam: A Novel Fungicide for Wheat.[Reference: WebLink]The development of an efficient, low-cost synthesis of the novel wheat fungicide silthiofam (1) is described. Improvements to the original Discovery route allowed 300 kg of material to be prepared in two, overlapping pilot-plant campaigns. Thereafter, efforts were focused on further optimizing the pilot-plant route, and on devising alternate, lower cost routes to silthiofam.
|

Methyl 3-methoxyacrylate Dilution Calculator

Methyl 3-methoxyacrylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.6118 mL | 43.0589 mL | 86.1178 mL | 172.2356 mL | 215.2945 mL |
| 5 mM | 1.7224 mL | 8.6118 mL | 17.2236 mL | 34.4471 mL | 43.0589 mL |
| 10 mM | 0.8612 mL | 4.3059 mL | 8.6118 mL | 17.2236 mL | 21.5295 mL |
| 50 mM | 0.1722 mL | 0.8612 mL | 1.7224 mL | 3.4447 mL | 4.3059 mL |
| 100 mM | 0.0861 mL | 0.4306 mL | 0.8612 mL | 1.7224 mL | 2.1529 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 12-Hydroxyabietic acid
Catalog No.:BCN5284
CAS No.:3484-61-5
- Metiamide
Catalog No.:BCC1742
CAS No.:34839-70-8
- Bz-Tyr-Oet
Catalog No.:BCC3122
CAS No.:3483-82-7
- DL-Dithiothreitol
Catalog No.:BCC7586
CAS No.:3483-12-3
- 4'-Demethyleucomin
Catalog No.:BCN5283
CAS No.:34818-83-2
- Marilactone
Catalog No.:BCN7363
CAS No.:34818-17-2
- 8-Acetonyldihydroavicine
Catalog No.:BCN3303
CAS No.:348098-59-9
- BAY 57-1293
Catalog No.:BCC4050
CAS No.:348086-71-5
- Boc-Met(O)-OH
Catalog No.:BCC3425
CAS No.:34805-21-5
- H-Tyr(Bzl)-OMe.HCl
Catalog No.:BCC3132
CAS No.:34805-17-9
- H-D-Met-OH
Catalog No.:BCC2997
CAS No.:348-67-4
- Z-Asp(OBzl)-OH
Catalog No.:BCC2791
CAS No.:3479-47-8
- Optovin
Catalog No.:BCC6323
CAS No.:348575-88-2
- Coptisine
Catalog No.:BCN6320
CAS No.:3486-66-6
- Palmatine
Catalog No.:BCN5285
CAS No.:3486-67-7
- UK 383367
Catalog No.:BCC2308
CAS No.:348622-88-8
- Vineridine
Catalog No.:BCN5286
CAS No.:3489-06-3
- HC-030031
Catalog No.:BCC4910
CAS No.:349085-38-7
- GSK 137647
Catalog No.:BCC8045
CAS No.:349085-82-1
- JX 401
Catalog No.:BCC7443
CAS No.:349087-34-9
- Cryptomeridiol 11-rhamnoside
Catalog No.:BCN4648
CAS No.:349112-30-7
- S-Adenosyl-L-Methionine iodide salt
Catalog No.:BCN2232
CAS No.:3493-13-8
- Alda 1
Catalog No.:BCC6096
CAS No.:349438-38-6
- Methyl dodonate A acetate
Catalog No.:BCN4647
CAS No.:349487-98-5
Metabolism of methyl-(E)-2-[2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl]-3-methoxyacrylate (azoxystrobin) in rat.[Pubmed:12851042]
Xenobiotica. 2003 Jun;33(6):677-90.
1. The metabolic fate of [(14)C]-methyl-(E)-2-[2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl]-3-methoxyacr ylate (azoxystrobin) was determined in the male and female rat following a single oral dose of 1 and 100 mg x kg(-1) and in surgically prepared, bile duct-cannulated rats following a single oral dose of 100 mg x kg(-1). 2. Azoxystrobin was extensively metabolized with at least 15 metabolites. There was a sex difference, with females producing more metabolites than males. 3. The two principal metabolic pathways were hydrolysis of the methoxyacid followed by glucuronic acid conjugation and glutathione conjugation of the cyanophenyl ring followed by further metabolism leading to the mercapturic acid. There were also several other minor pathways.
MGM-9 [(E)-methyl 2-(3-ethyl-7a,12a-(epoxyethanoxy)-9-fluoro-1,2,3,4,6,7,12,12b-octahydro-8-methoxy indolo[2,3-a]quinolizin-2-yl)-3-methoxyacrylate], a derivative of the indole alkaloid mitragynine: a novel dual-acting mu- and kappa-opioid agonist with potent antinociceptive and weak rewarding effects in mice.[Pubmed:18550129]
Neuropharmacology. 2008 Aug;55(2):154-65.
Mitragynine is a major indole alkaloid isolated from the Thai medicinal plant Mitragyna speciosa that has opium-like properties, although its chemical structure is quite different from that of morphine. We attempted to develop novel analgesics derived from mitragynine, and thus synthesized the ethylene glycol-bridged and C10-fluorinated derivative of mitragynine, MGM-9 [(E)-methyl 2-(3-ethyl-7a,12a-(epoxyethanoxy)-9-fluoro-1,2,3,4,6,7,12,12b-octahydro-8-methoxy indolo[2,3-a]quinolizin-2-yl)-3-methoxyacrylate]. We hypothesized that a dual-acting mu- and kappa-opioid agonist could produce potent antinociceptive effects with fewer rewarding effects compared with mu agonists. In this study, MGM-9 exhibited high affinity for mu- and kappa-opioid receptors with Ki values of 7.3 and 18 nM, respectively. MGM-9 showed a potent opioid agonistic effect, and its effects were meditated by mu- and kappa-opioid receptor mechanisms in in vitro assays. Subcutaneous and oral administration of MGM-9 produced potent antinociceptive effects in mouse tail-flick, hot-plate, and writhing tests. When administered orally, the antinociceptive effect of MGM-9 was seven to 22 times more potent than that of morphine. The antinociceptive effects of MGM-9 were mediated by both mu- and kappa-opioid receptors. Subcutaneous administration of MGM-9 twice daily for 5 days led to antinociceptive tolerance. In the gastrointestinal transit study, MGM-9 inhibited gastrointestinal transit, but its effect was weaker than that of morphine at equi-antinociceptive doses. Furthermore, MGM-9 induced less hyperlocomotion and fewer rewarding effects than morphine. The rewarding effect of MGM-9 was blocked by a mu antagonist and enhanced by a kappa antagonist. Taken together, the results suggest that MGM-9 is a promising novel analgesic that has a stronger antinociceptive effect and weaker adverse effects than morphine.


