GR 135531High affinity melatonin MT3 ligand CAS# 190277-13-5 |
- GPR120 modulator 1
Catalog No.:BCC1599
CAS No.:1050506-75-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 190277-13-5 | SDF | Download SDF |
| PubChem ID | 4284525 | Appearance | Powder |
| Formula | C14H17N3O3 | M.Wt | 275.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 5-MCA-NAT | ||
| Solubility | Soluble to 100 mM in ethanol | ||
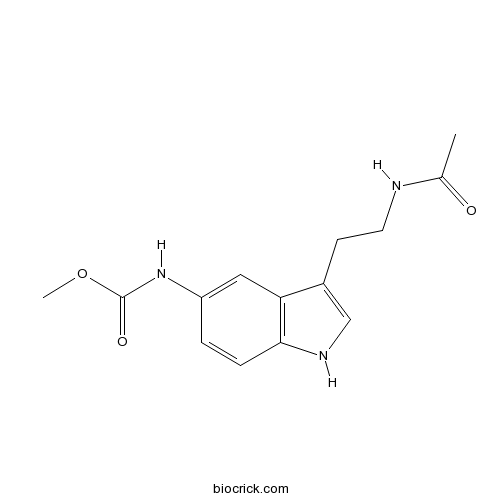
| Chemical Name | methyl N-[3-(2-acetamidoethyl)-1H-indol-5-yl]carbamate | ||
| SMILES | CC(=O)NCCC1=CNC2=C1C=C(C=C2)NC(=O)OC | ||
| Standard InChIKey | MPZVHKLZCUEJFO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H17N3O3/c1-9(18)15-6-5-10-8-16-13-4-3-11(7-12(10)13)17-14(19)20-2/h3-4,7-8,16H,5-6H2,1-2H3,(H,15,18)(H,17,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Binds with high affinity to MT3 melatonin sites in hamster brain, and low affinity (and no efficacy) at MT1 and MT2 melatonin receptors and thus can be used to discriminate between the melatonin receptor subtypes. |

GR 135531 Dilution Calculator

GR 135531 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6323 mL | 18.1613 mL | 36.3227 mL | 72.6454 mL | 90.8067 mL |
| 5 mM | 0.7265 mL | 3.6323 mL | 7.2645 mL | 14.5291 mL | 18.1613 mL |
| 10 mM | 0.3632 mL | 1.8161 mL | 3.6323 mL | 7.2645 mL | 9.0807 mL |
| 50 mM | 0.0726 mL | 0.3632 mL | 0.7265 mL | 1.4529 mL | 1.8161 mL |
| 100 mM | 0.0363 mL | 0.1816 mL | 0.3632 mL | 0.7265 mL | 0.9081 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bripiodionene
Catalog No.:BCN1831
CAS No.:190265-70-4
- Spiratisanin C
Catalog No.:BCN6975
CAS No.:1902173-22-1
- Spiratisanin B
Catalog No.:BCN6976
CAS No.:1902173-19-6
- Spiratisanin A
Catalog No.:BCN6977
CAS No.:1902173-16-3
- Demethoxyencecalin
Catalog No.:BCN1175
CAS No.:19013-07-1
- Eupatoriochromene
Catalog No.:BCN1174
CAS No.:19013-03-7
- 7-Hydroxy-2,2-dimethylchromene
Catalog No.:BCN7784
CAS No.:19012-97-6
- N-Acetyl-O-phosphono-Tyr-Glu Dipentylamide
Catalog No.:BCC5855
CAS No.:190078-50-3
- Firocoxib
Catalog No.:BCC5498
CAS No.:189954-96-9
- Mesopram
Catalog No.:BCC7549
CAS No.:189940-24-7
- Palmitoylisopropylamide
Catalog No.:BCC7187
CAS No.:189939-61-5
- 3-O-Feruloylquinic acid
Catalog No.:BCN3353
CAS No.:1899-29-2
- 7-Hydroxy-4-Methyl-8-Nitrocoumarin
Catalog No.:BCC9210
CAS No.:19037-69-5
- Sarracine N-oxide
Catalog No.:BCN2022
CAS No.:19038-27-8
- 3-Butyryloxytropane
Catalog No.:BCN1924
CAS No.:19038-34-7
- Orientanol A
Catalog No.:BCN4064
CAS No.:190381-82-9
- SLIGKV-NH2
Catalog No.:BCC3959
CAS No.:190383-13-2
- 17β-Benzoyloxy-androsta-1,4-dien-3-one
Catalog No.:BCC8443
CAS No.:19041-66-8
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
- AT 1015
Catalog No.:BCC6194
CAS No.:190508-50-0
- Dioscin
Catalog No.:BCN6273
CAS No.:19057-60-4
- Prosapogenin A
Catalog No.:BCN2582
CAS No.:19057-67-1
- Ac-Gly-OEt
Catalog No.:BCC2944
CAS No.:1906-82-7
- Ro 32-3555
Catalog No.:BCC2377
CAS No.:190648-49-8
Synthesis of new C-25 and C-26 steroidal acids as potential ligands of the nuclear receptors DAF-12, LXR and GR.[Pubmed:28300583]
Steroids. 2017 May;121:40-46.
A new methodology to obtain C-25 and C-26 steroidal acids starting from pregnenolone is described. Construction of the side chain was achieved by applying the Mukaiyama aldol reaction with a non-hydrolytic work-up to isolate the trapped silyl enol ether with higher yields. Using this methodology we synthesized three new steroidal acids as potential ligands of DAF-12, Liver X and Glucocorticoid nuclear receptors and studied their activity in reporter gene assays. Our results show that replacement of the 21-CH3 by a 20-keto group in the side chains of the cholestane scaffold of DAF-12 or Liver X receptors ligands causes the loss of the activity.
Identification of the Clinical Candidate (R)-(1-(4-Fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexah ydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methano ne (CORT125134): A Selective Glucocorticoid Receptor (GR) Antagonist.[Pubmed:28368581]
J Med Chem. 2017 Apr 27;60(8):3405-3421.
The nonselective glucocorticoid receptor (GR) antagonist mifepristone has been approved in the U.S. for the treatment of selected patients with Cushing's syndrome. While this drug is highly effective, lack of selectivity for GR leads to unwanted side effects in some patients. Optimization of the previously described fused azadecalin series of selective GR antagonists led to the identification of CORT125134, which is currently being evaluated in a phase 2 clinical study in patients with Cushing's syndrome.
Recruitment of bone marrow CD11b(+)Gr-1(+) cells by polymeric nanoparticles for antigen cross-presentation.[Pubmed:28317931]
Sci Rep. 2017 Mar 20;7:44691.
The objective of this study was to investigate the function of poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) on the activation of antigen-specific CD8(+) T cell responses via the CD11b(+)Gr(-)1(+) myeloid subpopulations in murine bone marrow (BM). PLGA NPs containing ovalbumin (OVA) were fabricated by the double-emulsion method. The CD11b(+)Gr-1(low)Ly-6C(high) and CD11b(+)Gr-1(high)Ly-6C(low) subsets from mice bone marrow were sorted and treated with the PLGA/OVA NPs, followed by co-culture with the carboxyfluorescein succinimidyl ester (CFSE)-labelled OT-I CD8(+) cells. Co-culture of OT-I CD8(+) T cells with PLGA/OVA NPs-primed CD11b(+)Gr-1(+) subsets upregulated the expression of IL-2, TNF-alpha, INF-gamma, granzyme B, and perforin, resulting in proliferation of CD8(+) T cells and differentiation into effector cytotoxic T lymphocytes (CTLs). In vivo proliferation of CFSE-labelled OT-I CD8(+) cells in response to OVA was also obtained in the animals immunized with PLGA/OVA NPs. The results presented in this study demonstrate the ability of polymeric NPs to recruit two CD11b(+)Gr(-)1(+) myeloid subsets for effective presentation of exogenous antigen to OT-I CD8(+) T cells in the context of major histocompatibility complex (MHC) class I, leading to an induction of antigen-specific cell proliferation and differentiation into effector cells.
Muscle-specific downregulation of GR levels inhibits adipogenesis in porcine intramuscular adipocyte tissue.[Pubmed:28360421]
Sci Rep. 2017 Mar 30;7(1):510.
Intramuscular adipose is conducive to good pork quality, whereas subcutaneous adipose is considered as waste in pig production. So uncovering the regulation differences between these two adiposes is helpful to tissue-specific control of fat deposition. In this study, we found the sensitivity to glucocorticoids (GCs) was lower in intramuscular adipocytes (IMA) compared with subcutaneous adipocytes (SA). Comparison of glucocorticoid receptor (GR) revealed that IMA had lower GR level which contributed to its reduced GCs sensitivity. Higher methylation levels of GR promotor 1-C and 1-H were detected in IMA compared with SA. GR expression decrease was also found in adipocytes when treated with muscle conditioned medium (MCM) in vitro, which resulted in significant inhibition of adipocytes proliferation and differentiation. Since abundant myostatin (MSTN) was detected in MCM by ELISA assay, we further investigated the effect of this myokine on adipocytes. MSTN treatment suppressed adipocytes GR expression, cell proliferation and differentiation, which mimicked the effects of MCM. The methylation levels of GR promotor 1-C and 1-H were also elevated after MSTN treatment. Our study reveals the role of GR in muscle fiber inhibition on intramuscular adipocytes, and identifies myostatin as a muscle-derived modulator for adipose GR level.
Investigation into the contractile response of melatonin in the guinea-pig isolated proximal colon: the role of 5-HT4 and melatonin receptors.[Pubmed:9283717]
Br J Pharmacol. 1997 Aug;121(8):1775-81.
1. The interaction of melatonin (N-acetyl-5-methoxytryptamine) with 5-hydroxytryptamine4 (5-HT4) receptors and/or with melatonin receptors (ML1, ML2 sites) has been assessed in isolated strips of the guinea-pig proximal colon. In the same preparation, the pharmacological profile of a series of melatonin agonists (2-iodomelatonin, 6-chloromelatonin, N-acetyl-5-hydroxytryptamine (N-acetyl-5-HT), 5-methoxycarbonylamino-N-acetyltryptamine (5-MCA-NAT)) was investigated. 2. In the presence of 5-HT1/2/3 receptor blockade with methysergide (1 microM) and ondansetron (10 microM), melatonin (0.1 nM-10 microM), 5-HT (1 nM-1 microM) and the 5-HT4 receptor agonist, 5-methoxytryptamine (5-MeOT: 1 nM-1 microM) caused concentration-dependent contractile responses. 5-HT and 5-MeOT acted as full agonists with a potency (-log EC50) of 7.8 and 8.0, respectively. The potency value for melatonin was 8.7, but its maximum effect was only 58% of that elicited by 5-HT. 3. Melatonin responses were resistant to atropine (0.1 microM), tetrodotoxin (0.3 microM), and to blockade of 5-HT4 receptors by SDZ 205,557 (0.3 microM) and GR 125487 (3, 30 and 300 nM). The latter antagonist (3 nM) inhibited 5-HT-induced contractions with an apparent pA2 value of 9.6 GR 125487 antagonism was associated with 30% reduction of the 5-HT response maximum. Contractions elicited by 5-HT were not modified when melatonin (1 and 10 nM) was used as an antagonist. 4. Like melatonin, the four melatonin analogues concentration-dependently contracted colonic strips. The rank order of agonist potency was: 2-iodomelatonin (10.8) > 6-chloromelatonin (9.9) > or = N-acetyl-5-HT (9.8) > or = 5-MCA-NAT (9.6) > melatonin (8.7), an order typical for ML2 sites. In comparison with the other agonists, 5-MCA-NAT had the highest intrinsic activity. 5. The melatonin ML1B receptor antagonist luzindole (0.3, 1 and 3 microM) had no effect on the concentration-response curve to melatonin. Prazosin, an alpha-adrenoceptor antagonist possessing moderate/ high affinity for melatonin ML2 sites did not affect melatonin-induced contractions at 0.1 microM. Higher prazosin concentrations (0.3 and 1 microM) caused a non-concentration-dependent depression of the maximal response to melatonin without changing its potency. Prazosin (0.1 and 1 microM) showed a similar depressant behaviour towards the contractile responses to 5-MCA-NAT. 6. In the guinea-pig proximal colon, melatonin despite some structural similarity with the 5-HT4 receptor agonist 5-MeOT, does not interact with 5-HT4 receptors (or with 5-HT1/2/3 receptors). As indicated by the rank order of agonist potencies and by the inefficacy of luzindole, the most likely sites of action of melatonin are postjunctional ML2 receptors. However, this assumption could not be corroborated with the use of prazosin as this 'ML2 receptor antagonist' showed only a non-concentration-dependent depression of the maximal contractile response to both melatonin and 5-MCA-NAT. Further investigation with the use of truly selective antagonists at melatonin ML2 receptors is required to clarify this issue.


