EupatoriochromeneCAS# 19013-03-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19013-03-7 | SDF | Download SDF |
| PubChem ID | 100768 | Appearance | Cryst. |
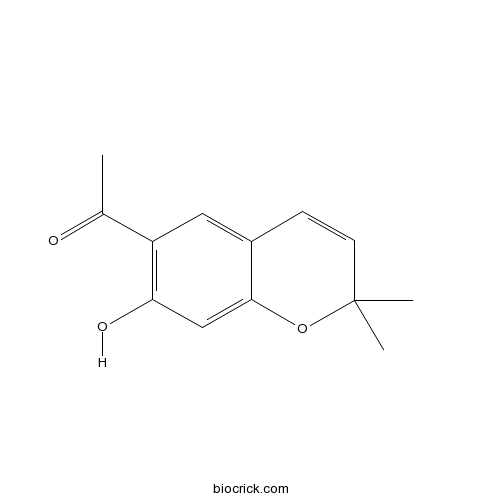
| Formula | C13H14O3 | M.Wt | 218.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(7-hydroxy-2,2-dimethylchromen-6-yl)ethanone | ||
| SMILES | CC(=O)C1=C(C=C2C(=C1)C=CC(O2)(C)C)O | ||
| Standard InChIKey | SVUVYHFYZBCYRF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H14O3/c1-8(14)10-6-9-4-5-13(2,3)16-12(9)7-11(10)15/h4-7,15H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Eupatoriochromene retard seed germination , reduced radicle and hypocotyl growth of weed and crop plant seedlings and increased adventitious root formation of mung bean cuttings. 2. Eupatoriochromene has insecticidal activity, it exhibits toxicity againstCulex pipiens (house mosquito) larvae andOncopeltus fasciatus (large milkweed bug) nymphs. |
| Targets | Antifection |

Eupatoriochromene Dilution Calculator

Eupatoriochromene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5809 mL | 22.9043 mL | 45.8085 mL | 91.617 mL | 114.5213 mL |
| 5 mM | 0.9162 mL | 4.5809 mL | 9.1617 mL | 18.3234 mL | 22.9043 mL |
| 10 mM | 0.4581 mL | 2.2904 mL | 4.5809 mL | 9.1617 mL | 11.4521 mL |
| 50 mM | 0.0916 mL | 0.4581 mL | 0.9162 mL | 1.8323 mL | 2.2904 mL |
| 100 mM | 0.0458 mL | 0.229 mL | 0.4581 mL | 0.9162 mL | 1.1452 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7-Hydroxy-2,2-dimethylchromene
Catalog No.:BCN7784
CAS No.:19012-97-6
- N-Acetyl-O-phosphono-Tyr-Glu Dipentylamide
Catalog No.:BCC5855
CAS No.:190078-50-3
- Firocoxib
Catalog No.:BCC5498
CAS No.:189954-96-9
- Mesopram
Catalog No.:BCC7549
CAS No.:189940-24-7
- Palmitoylisopropylamide
Catalog No.:BCC7187
CAS No.:189939-61-5
- 3-O-Feruloylquinic acid
Catalog No.:BCN3353
CAS No.:1899-29-2
- 8-Amino-2-methylquinoline
Catalog No.:BCC8782
CAS No.:18978-78-4
- Ro 10-5824 dihydrochloride
Catalog No.:BCC7330
CAS No.:189744-94-3
- Akuammiline
Catalog No.:BCN4772
CAS No.:1897-26-3
- 6,4'-Dihydroxy-7-methoxyflavanone
Catalog No.:BCN7797
CAS No.:189689-32-5
- trans-4-phenylbut-3-en-2-one
Catalog No.:BCN3805
CAS No.:1896-62-4
- Dihydrooroxylin A
Catalog No.:BCN3500
CAS No.:18956-18-8
- Demethoxyencecalin
Catalog No.:BCN1175
CAS No.:19013-07-1
- Spiratisanin A
Catalog No.:BCN6977
CAS No.:1902173-16-3
- Spiratisanin B
Catalog No.:BCN6976
CAS No.:1902173-19-6
- Spiratisanin C
Catalog No.:BCN6975
CAS No.:1902173-22-1
- Bripiodionene
Catalog No.:BCN1831
CAS No.:190265-70-4
- GR 135531
Catalog No.:BCC6836
CAS No.:190277-13-5
- 7-Hydroxy-4-Methyl-8-Nitrocoumarin
Catalog No.:BCC9210
CAS No.:19037-69-5
- Sarracine N-oxide
Catalog No.:BCN2022
CAS No.:19038-27-8
- 3-Butyryloxytropane
Catalog No.:BCN1924
CAS No.:19038-34-7
- Orientanol A
Catalog No.:BCN4064
CAS No.:190381-82-9
- SLIGKV-NH2
Catalog No.:BCC3959
CAS No.:190383-13-2
- 17β-Benzoyloxy-androsta-1,4-dien-3-one
Catalog No.:BCC8443
CAS No.:19041-66-8
Insecticidal chromenes from the volatile oil ofHemizonia fitchii.[Pubmed:24310216]
J Chem Ecol. 1985 Jun;11(6):701-12.
Based on field observations of the effects of the resinous tarweedHemizonia fitchii A. Gray (Asteraceae) on mosquito populations in California, the volatile oil of this plant was investigated for insecticidal activity. Analysis of the oil by TLC and capillary GC-MS showed the presence of five major constituents which were identified as the monoterpenoid 1,8-cineole, and the chromenes encecalin, Eupatoriochromene (desmethylencecalin), 6-vinyl-7-methoxy-2,2-dimethylchromene, and desmethoxyencecalin. Trace amounts of several volatile fatty acids, alkanes,p-coumarate derivatives, additional chromene derivatives, and numerous mono- and sesquiterpenoids were also detected and identified by GC-MS. Fractionation of the oil by preparative TLC and column chromatography afforded the major chromenes, the identities of which were confirmed by NMR and IR spectral data. The chromenes exhibited weak to moderate toxicity againstCulex pipiens (house mosquito) larvae andOncopeltus fasciatus (large milkweed bug) nymphs. However, no antijuvenile hormone activity was observed for any of the compounds tested against these insect species.
Eupatoriochromene and encecalin, plant growth regulators from yellow starthistle (Centaurea solstitialis L.).[Pubmed:24272297]
J Chem Ecol. 1989 Jul;15(7):2073-87.
Two chromenes, Eupatoriochromene (1) and encecalin (2), have been isolated from yellow starthistle (Centaurea solstitialis L.). Both chromenes retard seed germination and reduce radicle and hypocotyl growth of weed and crop plant seedlings. In addition,1 increases adventitious root formation of mung bean cuttings.


