FlumethasoneCAS# 2135-17-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2135-17-3 | SDF | Download SDF |
| PubChem ID | 16490 | Appearance | Powder |
| Formula | C22H28F2O5 | M.Wt | 410.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Flumetasone | ||
| Solubility | DMSO : 100 mg/mL (243.64 mM; Need ultrasonic) | ||
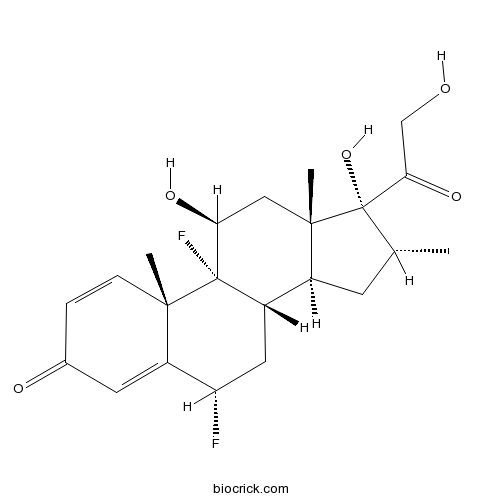
| Chemical Name | (6S,8S,9R,10S,11S,13S,14S,16R,17R)-6,9-difluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one | ||
| SMILES | CC1CC2C3CC(C4=CC(=O)C=CC4(C3(C(CC2(C1(C(=O)CO)O)C)O)F)C)F | ||
| Standard InChIKey | WXURHACBFYSXBI-GQKYHHCASA-N | ||
| Standard InChI | InChI=1S/C22H28F2O5/c1-11-6-13-14-8-16(23)15-7-12(26)4-5-19(15,2)21(14,24)17(27)9-20(13,3)22(11,29)18(28)10-25/h4-5,7,11,13-14,16-17,25,27,29H,6,8-10H2,1-3H3/t11-,13+,14+,16+,17+,19+,20+,21+,22+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Flumethasone Dilution Calculator

Flumethasone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4361 mL | 12.1803 mL | 24.3605 mL | 48.7211 mL | 60.9013 mL |
| 5 mM | 0.4872 mL | 2.4361 mL | 4.8721 mL | 9.7442 mL | 12.1803 mL |
| 10 mM | 0.2436 mL | 1.218 mL | 2.4361 mL | 4.8721 mL | 6.0901 mL |
| 50 mM | 0.0487 mL | 0.2436 mL | 0.4872 mL | 0.9744 mL | 1.218 mL |
| 100 mM | 0.0244 mL | 0.1218 mL | 0.2436 mL | 0.4872 mL | 0.609 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Flumethasone is a corticosteroid for topical use, in combination with Clioquinol for the treatment of otitis externa and otomycosis. Flumethasone shows fully 420 times the potency of cortisone in an animal model for anti-inflammatory activity.
- Ercalcidiol
Catalog No.:BCC1555
CAS No.:21343-40-8
- 1-Methyl-L-tryptophan
Catalog No.:BCN8341
CAS No.:21339-55-9
- 18-Nor-4,15-dihydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1494
CAS No.:213329-46-5
- 15,18-Dihydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1495
CAS No.:213329-45-4
- H-Pro-OMe.HCl
Catalog No.:BCC3022
CAS No.:2133-40-6
- RITA (NSC 652287)
Catalog No.:BCC2238
CAS No.:213261-59-7
- [Phe1Ψ(CH2-NH)Gly2]Nociceptin(1-13)NH2
Catalog No.:BCC5701
CAS No.:213130-17-7
- Ceanothic acid
Catalog No.:BCN4918
CAS No.:21302-79-4
- Org 37684
Catalog No.:BCC6291
CAS No.:213007-95-5
- Boc-Tyr(Bzl)-OH
Catalog No.:BCC3461
CAS No.:2130-96-3
- Tetrahymanol acetate
Catalog No.:BCN6933
CAS No.:2130-22-5
- Tetrahymanol
Catalog No.:BCN6934
CAS No.:2130-17-8
- Drim-7-ene-11,12-diol acetonide
Catalog No.:BCN4919
CAS No.:213552-47-7
- 5-Iodo-A-85380, 5-trimethylstannyl N-BOC derivative
Catalog No.:BCC7102
CAS No.:213766-21-3
- 6,8-Cyclo-1,4-eudesmanediol
Catalog No.:BCN4920
CAS No.:213769-80-3
- Zedoarofuran
Catalog No.:BCN3527
CAS No.:213833-34-2
- Nifenazone
Catalog No.:BCC3822
CAS No.:2139-47-1
- 1-Acetoxy-9,17-octadecadiene-12,14-diyne-11,16-diol
Catalog No.:BCN1493
CAS No.:213905-35-2
- Barbacarpan
Catalog No.:BCN4921
CAS No.:213912-46-0
- Chrysin dimethylether
Catalog No.:BCN6799
CAS No.:21392-57-4
- Taxiphyllin
Catalog No.:BCN4922
CAS No.:21401-21-8
- CART (55-102) (human)
Catalog No.:BCC6007
CAS No.:214050-22-3
- Magnoflorine
Catalog No.:BCN4923
CAS No.:2141-09-5
- Glucoraphanin
Catalog No.:BCN3817
CAS No.:21414-41-5
Environmental glucocorticoids corticosterone, betamethasone and flumethasone induce more potent physiological than transcriptional effects in zebrafish embryos.[Pubmed:30954817]
Sci Total Environ. 2019 Mar 28;672:183-191.
Many glucocorticoids occur in the aquatic environments but their adverse effects to fish are poorly known. Here we investigate effects of the natural glucocorticoid corticosterone and the synthetic glucocorticoids betamethasone and Flumethasone in zebrafish embryos. Besides studying the effects of each steroid, we compared effects of natural with synthetic glucocorticoids, used as drugs. Exposure at concentrations of 1mug/L and higher led to concentration-related decrease in spontaneous muscle contractions at 24h post fertilization (hpf) and increase in heart rate at 48hpf. Betamethasone showed a significant increase at 0.11mug/L in heart rate. Corticosterone also accelerated hatching at 60hpf at 0.085mug/L. Transcription of up to 24 genes associated with different pathways showed alterations at 96 and 120hpf for all glucocorticoids, although with low potency. Corticosterone caused transcriptional induction of interleukin-17, while betamethasone caused transcriptional down-regulation of the androgen receptor, aromatase and hsd11b2, indicating an effect on the sex hormone system. Furthermore, transcripts encoding proteins related to immune system regulation (irg1l, gilz) and fkbp5 were differentially expressed by corticosterone and betamethasone, while Flumethasone caused only little effects, mainly alteration of the irg1l transcript. Our study shows that these glucocorticoids caused more potent physiological effects in early embryos than transcriptional alterations in hatched embryos, likely due to increased metabolism in later developmental stages. Thus, these glucocorticoids may be of concern for early stages of fish embryos in contaminated aquatic environments.
Interventions for infantile seborrhoeic dermatitis (including cradle cap).[Pubmed:30828791]
Cochrane Database Syst Rev. 2019 Mar 4;3:CD011380.
BACKGROUND: Infantile seborrhoeic dermatitis (ISD) is a chronic, inflammatory, scaling skin condition, which causes redness and a greasy scaling rash in infants and young children. It can last from weeks to months, but rarely years. When it occurs on the scalp, it is referred to as 'cradle cap'. While benign and self-limiting, irrelevant of its location on the body, it can distress parents. The effectiveness of commonly promoted treatments is unclear. OBJECTIVES: To assess the effects of interventions for infantile seborrhoeic dermatitis in children from birth to 24 months of age. SEARCH METHODS: We searched the following databases up to 22 May 2018: Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We searched trials registers and checked reference lists of included studies for further references to randomised controlled trials (RCTs). We searched for unpublished RCTs and grey literature via web search engines, and wrote to authors and pharmaceutical companies. SELECTION CRITERIA: We included RCTs of interventions for ISD in children from birth up to 24 months who were clinically diagnosed by a healthcare practitioner with ISD or cradle cap. We allowed comparison of any treatment to no treatment or placebo, and the comparison of two or more treatments or a combination of treatments. DATA COLLECTION AND ANALYSIS: We used standard methodological procedures expected by Cochrane. The primary outcome measures were 'Change in severity score from baseline to end of study' and 'Percentage of infants treated who develop adverse effects or intolerance to treatment'. The secondary outcome was 'Improvement in quality of life (QoL) as reported by parents'. MAIN RESULTS: We included six RCTs (one with a cross-over design) randomising 310 children and reporting outcomes for 297 children. Most participants were aged under seven months with only two participants aged over one year (seven and 12 years old); where specified, 60% were boys. In two studies, condition severity was mild to moderate; one study included two participants with severe ISD; the other studies did not describe baseline severity or described it as body surface area affected.The study setting was not always clear but likely a paediatric outpatient clinic in the following countries: Thailand, Israel, USA, France, and Australia.Two studies compared oral biotin (a B group vitamin) against placebo, two studies compared proprietary products against placebo cream or a control shampoo, and two studies compared topical corticosteroids against other products. The studies were generally short-term, between 10 and 42 days' duration; only one study followed the participants until resolution of the rash or eight months of age.We assessed the risk of bias as unclear for most aspects due to lack of reporting, but two of the studies were at high risk of performance and detection bias due to the appearance of the intervention, the trial design (open-label), or use of overlabelled tubes. Two trials had a high risk of attrition bias.All the results given below were based on very low-quality evidence. Treatment duration ranged from one week to three weeks.For the two trials comparing biotin versus placebo (n = 35), one did not report a measure of change in severity (only change in duration of rash) while the other did not report raw data (only 'no statistically significant difference'), measured at three weeks. Neither trial reported on adverse events.Two trials compared proprietary products against placebo (n = 160). One trial assessed change in severity via percentage success (96% of participants in non-steroidal cream Promiseb versus 92% in placebo), and reported no adverse events (both assessed at day 14). The other trial assessed change in severity via reduction in lesional score (surface area covered), finding better results for lactamide MEA gel (a moisturising agent) plus shampoo (81.4%) compared with shampoo only (70.2%; P = 0.0092). No adverse events were described, but signs of discomfort were similar in both groups (both assessed at day 21).In the comparison of topical steroids versus another product, change in severity was measured through evaluation of cure and body surface (n = 102).In one trial comparing hydrocortisone 1% lotion with licochalcone 0.025% lotion, there was no significant difference in participants cured (95.8% with hydrocortisone compared to 97.1% with licochalcone). One person in the licochalcone group developed more erythema, but there were no other adverse events (both outcomes assessed at day 14). In the trial comparing Flumethasone pivalate 0.02% ointment versus eosin 2% aqueous solution, a reduction in body surface area affected was seen in both groups at day 10 (9% with corticosteroid versus 7% with aqueous solution), with all infants showing less than 10% involvement. There were no adverse events (both outcomes assessed at day 10).No studies measured QoL.We found no trials testing commonly used treatments such as mineral oils, salicylic acid, or antifungals. AUTHORS' CONCLUSIONS: Our review identified only a limited number of studies investigating the effects of interventions for ISD in infants and young children. Unlike the reviews investigating the effects of treatments in adults, our results showed that there is uncertainty regarding the effectiveness and safety of studied treatments due to the very low-certainty evidence for all comparisons and outcomes.We assessed most bias domains as at unclear risk, but there was a high risk of bias for (mainly) performance, attrition, and detection bias. Evidence was limited further by imprecision (small studies, low number of events), indirectness (mainly with the outcomes assessed), and poor trial reporting. In most studies, the prognosis for the condition was favourable regardless of intervention but interpretation is limited by the very low-certainty evidence.Further research is needed with large, well-conducted, and well-reported intervention trials, particularly of interventions commonly recommended or used, such as emollients or shampoos and brushing, antifungals, or steroids. All studies should report standardised and validated relevant outcome measures, including adverse events, severity, and QoL, and they should be conducted in primary care settings where the majority of ISD is managed. Future trials should compare against placebo, no treatment, or standard care.
GR CALUX assay detects synthetic glucocorticoids in calf urine: a validation study.[Pubmed:30702391]
Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2019 Mar;36(3):413-423.
Member States of the EU are required to monitor the use of pharmacologically active substances in food-producing animals. There is evidence, however, that the target-based approach currently applied in official monitoring plans might under-estimate the real incidence of growth promoter abuse in livestock. As demonstrated for sex hormones, the association of effect-oriented biological screening with chemical confirmatory techniques could be the best strategy in revealing the abuse of veterinary drugs. Here we demonstrate the reliability of a cell-based assay to screen calf urine samples for synthetic glucocorticoids. The validation included the most widely used synthetic drugs (Flumethasone, dexamethasone, betamethasone, methylprednisolone and prednisolone) and was developed according to the Commission Decision 2002/657/EC, thus including the verification of cut-off level, the beta error, the specificity, ruggedness and stability. The study was carried out using prednisolone as representative substance at 5 ng mL(-1) concentration. All blank and spiked urine fulfilled the EU criteria, moreover the method resulted in being specific and sound, and the analytes in urine were stable for at least 30 days. The assay results indicated its suitability for a qualitative analysis of calf urine samples. This method enabled the detection of low doses of synthetic glucocorticoids (GCs) in matrix (<2 ng mL(-1) for Flumethasone, dexamethasone, betamethasone; < 4 ng mL(-1) for methylprednisolone; 5 ng mL(-1) for prednisolone), with the possibility of detecting new or unknown molecules and cumulative effects of low-level mixtures with glucocorticoid bioactivity.
Analysis of corticosteroids in samples of animal origin using QuEChERS and ultrahigh-performance liquid chromatography coupled to high-resolution mass spectrometry.[Pubmed:30478515]
Anal Bioanal Chem. 2019 Jan;411(2):449-457.
A rapid and sensitive method for the confirmatory analysis of eight synthetic corticosteroids (betamethasone, dexamethasone, prednisolone, 6-methylprednisolone, triamcinolone, Flumethasone, beclomethasone, fluocinolone acetonide) is proposed. The method is useful for detecting illegal treatments in different animal species. It consists of an extraction and cleanup using the quick, easy, cheap, effective, rugged, and safe (QuEChERS) strategy. Quantitative determination is achieved by ultrahigh-performance liquid chromatography coupled to high-resolution mass spectrometry with heated electrospray ionization in negative mode. Quantification is performed using surrogate matrix-matched standard calibration curve with dexamethasone-D4 as the internal standard. The method was validated for analyzing liver samples according to the criteria established by Decision 2002/657/EC. Linearity was assessed in the 1-10 mug kg(-1) range and linear correlation coefficients were over 0.99 for all the analytes. CCalpha ranged from 0.04 to 0.16 mug kg(-1) for substances without maximum residue limit. The method allows confident quantification and confirmation of corticosteroids in liver samples, and its simplicity makes it suitable for analyzing large numbers of samples.
Inhibitory effect of timolol on topical glucocorticoidinduced skin telangiectasia.[Pubmed:30015958]
Mol Med Rep. 2018 Sep;18(3):2823-2831.
The aim of the present study wasto investigate the potential inhibitory effect of timolol on topical glucocorticoidinduced skin telangiectasia. In rabbits, Flumethasone ointment was used to induce skin telangiectasia in the inner ear. Subsequently, timolol maleate (0.5%) eye drops (TMEDs) were administered twice daily for 4 weeks. Expression of the antibacterial peptides 37amino acid peptide (LL37) and kallikrein5 (KLK5) was detected using quantitative polymerase chain reaction (PCR) and semiquantitative reverse transcriptionPCR. In patients with facial skin telangiectasia, one cheek of each patient was assigned to a treatment group and the other to a control group. For the treatment group cheeks, topical application of TMEDs was combined with 0.1% tacrolimus ointment once or twice daily for 8 weeks. The control group cheeks were administered with 0.1% tacrolimus ointment alone. Alterations in lesions were recorded by dermoscopy, and the L, a and b values of lesions were measured, based on the Commission Internationale de l'Eclairage system, with a chromameter prior to and at 1, 2, 4 and 8 weeks following treatment. The results indicated that erythema, papules and telangiectasia were significantly diminished following 4 weeks of treatment with TMEDs in rabbits. Notably, the expression of LL37 and KLK5 mRNA was increased in the negative control group; however, it was decreased in the trial and blank groups. Clinical and dermoscopy images demonstrated that erythema was reduced in the 2 groups for 1 week, and that telangiectasia in the treatment group was markedly reduced compared with the control group at 4 weeks. The difference of the L and a values of lesions between the treatment and control group was significant (P<0.05). Overall, the present results suggested that the abnormal expression of LL37 may be one of the mechanisms underlying the pathogenesis of facial corticosteroid addiction dermatitis (FCAD) and TMEDs may inhibit the mRNA expression of LL37 by downregulating KLK5; in this regard, TMEDs may serve a role in attenuating telangiectasia, which may be beneficial in improving the telangiectasia symptoms of FCAD.
Occurrence of Glucocorticoids, Mineralocorticoids, and Progestogens in Various Treated Wastewater, Rivers, and Streams.[Pubmed:29580053]
Environ Sci Technol. 2018 May 1;52(9):5296-5307.
In the current study a high sensitive analytical method was developed for the determination of 60 steroids including glucocorticoids (GC), mineralocorticoids (MC), and progestogens (PG) in WWTP effluents and surface water using liquid chromatography with tandem mass spectrometry detection (LC-MS/MS). The limits of quantification (LOQ) ranged between 0.02 ng/L (cortisone) to 0.5 ng/L (drospirenone) in surface water and from 0.05 ng/L (betamethasone) to 5 ng/L (chlormadinone) in treated wastewater. After optimization, the developed method was applied to WWTP effluents, rivers, and streams around Germany. Numerous steroids have been detected during the sampling campaign and predominant analytes from all steroid types were determined. Moreover, the occurrence of dienogest, mometasone furoate, Flumethasone pivalate, and the metabolites 6beta-hydroxy dienogest, 6beta-hydroxy triamcinolone acetonide, 7alpha-thiomethyl spironolactone, and 11alpha-hydroxy canrenone is reported for the first time. In addition, this study revealed the ubiquitous presence of topically applied GC monoesters betamethasone propionate, betamethasone valerate, and 6alpha-methylprednisolone propionate in WWTP effluents and surface water.
[Clinical efficacy of ozonated oil in the treatment of psoriasis vulgaris].[Pubmed:29559602]
Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018 Feb 28;43(2):173-178.
OBJECTIVE: To compare the efficacy and safety between ozonated oil and compound Flumethasone ointment in the treatment of psoriasis vulgaris. Methods: A left/right self-controlled, parallel group study was conducted. Forty patients with stable psoriasis vulgaris were enrolled in the study, whose lesions were symmetrical and involvement areas were <30% body surface. The patients were divided into 2 groups. Patients with left lesions served as a test group were treated daily for ozonated oil twice, and patients with right lesions served as a control group were treated daily for compound flumetasone ointment twice. The patients in the 2 groups were treated for 4 weeks. The clinical efficacy and safety were observed at 1, 2 and 4 weeks after the treatment. Results: After 1 week treatment, the effective rates of the test group and the control group were 60.58% and 72.28%, respectively, with significant difference between them (P<0.05). At 2 weeks and 4 weeks after the treatment, the efficacy in the test group was similar to that in the control group. The effective rates in the test group and the control group were 69.84% and 70.25% after 2 weeks, respectively, 70.88% and 71.23% after 4 weeks, respectively. There was no significant difference between the 2 groups (P<0.05). In addition, the reflectance confocal microscope results in both the test group and the control group after 4 weeks showed that the epidermis was approximately normal. There were few inflammatory cells infiltration in the dermal papilla, and the inflammatory cells infiltration was significantly reduced after treatment. Conclusion: Ozonated oil treatment for stable psoriasis is safe and effective, and its efficacy is equivalent to the effect of glucocorticoid topical preparations.
A novel and innovative hair test to determine glucocorticoid levels in racing camels for use in assessment of doping, health, and disease.[Pubmed:28994213]
Drug Test Anal. 2018 Apr;10(4):742-749.
The aim of this project was to develop and validate a new test for the analysis of glucocorticoids in camel hair and to use the new test to analyse hair samples from a variety of camel breeds in sports and racing applications. These findings could be of importance when evaluating racing camels for suspected doping offenses or for injury and disease control. Camel hair samples were collected from 30 non-racing dromedary camels along with 3 racing camels in Al Ain, UAE and were decontaminated, pulverised, sonicated, and extracted prior to analysis. A liquid chromatographic-mass spectrometric method was employed to determine the levels of glucocorticoids in the hair samples. The 4 drugs of interest, namely hydrocortisone, dexamethasone, Flumethasone and methylprednisolone, and an internal standard were quantified in camel hair samples. All 4 of the glucocorticoids were detected in camel hair samples with concentrations ranging between 31 and 935 pg/mg for hydrocortisone, 8-59 pg/mg for dexamethasone, 0.7-1034 pg/mg for Flumethasone and 5-66 pg/mg for methylprednisolone in non-racing camels. One of the racing camels displayed high concentrations of hydrocortisone (1130 pg/mg), Flumethasone (2576 pg/mg), methylprednisone (1156 pg/mg) and dexamethasone (29 pg/mg). The authors believe this is the first report of a test for corticosteroids in camel hair. The new test has been validated according to Food and Drug Administration (FDA) guidelines. This new hair test could be useful for further studies in doping control, toxicological studies, pharmacological studies and other clinical applications in camel health, injury, and disease.
Multiresidue analysis of glucocorticoids in milk by LC-MS/MS with low-temperature purification and dispersive solid-phase extraction.[Pubmed:28556446]
J Sep Sci. 2017 Jul;40(13):2759-2768.
A multiresidue method for the determination of 12 glucocorticoids (clobetasol propionate, budesonide, triamcinolone, triamcinolone acetonide, fludrocortisone acetate, Flumethasone, beclomethasone, prednisone acetate, 6-alpha-methylprednisolone, hydrocortisone, cortisone, and prednisone) in bovine milk was developed using liquid chromatography with tandem mass spectrometry. Isoflupredone was used as an internal standard. Milk samples were treated with ethyl acetate to extract glucocorticoids and were frozen at -20 degrees C for 6 h to precipitate fat. The extract was dried under nitrogen, and residues were dissolved in an acetonitrile/water solution. A further clean-up step was used by dispersive solid-phase extraction, with octadecyl silica and primary secondary amine as the absorbents. The recoveries of glucocorticoids spiked at 0.5, 1.0, 10.0 mug/kg ranged from 75.7 to 117.3%, except for clobetasol propionate and budesonide (16.1-49.5%). The limits of quantification were 0.01-0.5 mug/kg in milk. This method has been successfully applied in real samples. The results demonstrated that this method is simple, robust, and suitable for identification of glucocorticoid residues in milk.
Simultaneous detection of flumethasone, dl-methylephedrine, and 2-hydroxy-4,6-dimethylpyrimidine in porcine muscle and pasteurized cow milk using liquid chromatography coupled with triple-quadrupole mass spectrometry.[Pubmed:26797492]
J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Feb 15;1012-1013:8-16.
A simple analytical method based on liquid chromatography coupled with triple-quadrupole mass spectrometry was developed for detection of the veterinary drugs Flumethasone, dl-methylephedrine, and 2-hydroxy-4,6-dimethylpyrimidine in porcine muscle and pasteurized cow milk. The target drugs were extracted from samples using 10mM ammonium formate in acetonitrile followed by clean-up with n-hexane and primary secondary amine sorbent (PSA). The analytes were separated on an XBridge hydrophilic interaction liquid chromatography (HILIC) column using 10mM ammonium formate in ultrapure water and acetonitrile. Good linearity was achieved over the tested concentrations in matrix-fortified calibrations with correlation coefficients (R(2))>/=0.9686. Recovery at two spiking levels ranged between 73.62-112.70% with intra- and inter-day precisions of
Synthesis and structure-activity relationships of novel cationic lipids with anti-inflammatory and antimicrobial activities.[Pubmed:26004577]
Bioorg Med Chem Lett. 2015 Jul 15;25(14):2837-43.
Certain membrane-active cationic steroids are known to also possess both anti-inflammatory and antimicrobial properties. This combined functionality is particularly relevant for potential therapies of infections associated with elevated tissue damage, for example, cystic fibrosis airway disease, a condition characterized by chronic bacterial infections and ongoing inflammation. In this study, six novel cationic glucocorticoids were synthesized using beclomethasone, budesonide, and Flumethasone. Products were either monosubstituted or disubstituted, containing one or two steroidal groups, respectively. In vitro evaluation of biological activities demonstrated dual anti-inflammatory and antimicrobial properties with limited cytotoxicity for all synthesized compounds. Budesonide-derived compounds showed the highest degree of both glucocorticoid and antimicrobial properties within their respective mono- and disubstituted categories. Structure-activity analyses revealed that activity was generally related to the potency of the parent glucocorticoid. Taken together, these data indicate that these types of dual acting cationic lipids can be synthesized with the appropriate starting steroid to tailor activities as desired.
Effect-based detection of synthetic glucocorticoids in bovine urine.[Pubmed:25569131]
Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2015;32(2):194-204.
Challenges to testing for the illicit use of anabolic substances in meat-producing animals stem from the production of new synthetic compounds and the administration of low-dose cocktails to circumvent detection by the surveillance schemes of European Union member states. This work evaluated for the first time GR-CALUX, a highly sensitive reporter gene assay, as a screening tool for the detection of synthetic glucocorticoids in bovine urine. In order to verify the effect of natural corticosteroids on the method, the bioassay was tested first using blank urine samples collected at the farm and the slaughterhouse. Next, the dose-response curves were measured for the most commonly used synthetic glucocorticoids. The bioassay's ability to detect them in spiked and incurred samples of bovine urine was then evaluated. Finally, its performance was compared against a commercially available ELISA kit ordinarily used in screening activities. GR-CALUX performance did not appear to be influenced by physiological levels of endogenous corticosteroids in the farm samples, whereas an increase in these hormones might invalidate the analysis in samples obtained at the slaughterhouse. Using pure compounds, GR-CALUX showed a high sensitivity toward the synthetic glucocorticosteroids tested in order of relative potencies: Flumethasone >> dexamethasone > betamethasone > methylprednisolone > prednisolone. As expected, the bioassay failed to detect the prohormone prednisone. The results obtained from analysis of the spiked and incurred specimens reproduced those of the blank samples and the pure compounds. GR-CALUX is a promising screening tool for the detection of illicit treatments in meat-producing bovines. Its ability to detect the most commonly used synthetic glucocorticoids was comparable with the ELISA test. Importantly, it appeared to be less susceptible to matrix effects than ELISA.
Novel spectrophotometric determination of flumethasone pivalate and clioquinol in their binary mixture and pharmaceutical formulation.[Pubmed:25448970]
Spectrochim Acta A Mol Biomol Spectrosc. 2015 Feb 5;136 Pt B:707-13.
This work is concerned with development and validation of three simple, specific, accurate and precise spectrophotometric methods for determination of Flumethasone pivalate (FP) and clioquinol (CL) in their binary mixture and ear drops. Method A is a ratio subtraction spectrophotometric one (RSM). Method B is a ratio difference spectrophotometric one (RDSM), while method C is a mean center spectrophotometric one (MCR). The calibration curves are linear over the concentration range of 3-45 mug/mL for FP, and 2-25 mug/mL for CL. The specificity of the developed methods was assessed by analyzing different laboratory prepared mixtures of the FP and CL. The three methods were validated as per ICH guidelines; accuracy, precision and repeatability are found to be within the acceptable limits.


