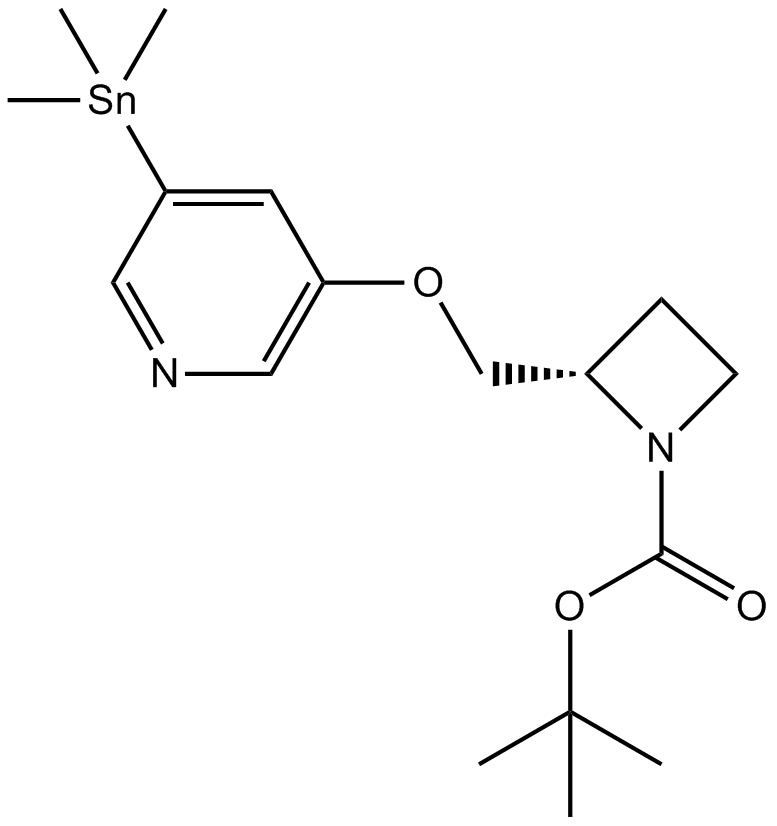
5-Iodo-A-85380, 5-trimethylstannyl N-BOC derivativeCAS# 213766-21-3 |
- Zebularine
Catalog No.:BCC1136
CAS No.:3690-10-6
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
- Nanaomycin A
Catalog No.:BCC3611
CAS No.:52934-83-5
- SGI-110
Catalog No.:BCC2221
CAS No.:929901-49-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 213766-21-3 | SDF | Download SDF |
| PubChem ID | 10574471 | Appearance | Powder |
| Formula | C17H28N2O3Sn | M.Wt | 427.11 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in ethanol and in DMSO | ||
| Chemical Name | tert-butyl (2S)-2-[(5-trimethylstannylpyridin-3-yl)oxymethyl]azetidine-1-carboxylate | ||
| SMILES | CC(C)(C)OC(=O)N1CCC1COC2=CC(=CN=C2)[Sn](C)(C)C | ||
| Standard InChIKey | HHNYNAVVSDTMQD-HZAYLZKLSA-N | ||
| Standard InChI | InChI=1S/C14H19N2O3.3CH3.Sn/c1-14(2,3)19-13(17)16-8-6-11(16)10-18-12-5-4-7-15-9-12;;;;/h5,7,9,11H,6,8,10H2,1-3H3;3*1H3;/t11-;;;;/m0..../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Precursor to the highly potent and selective ligand for the α4β2 nicotinic receptor, 5-Iodo-A-85380.Suitable for radioiodination. |

5-Iodo-A-85380, 5-trimethylstannyl N-BOC derivative Dilution Calculator

5-Iodo-A-85380, 5-trimethylstannyl N-BOC derivative Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3413 mL | 11.7066 mL | 23.4132 mL | 46.8263 mL | 58.5329 mL |
| 5 mM | 0.4683 mL | 2.3413 mL | 4.6826 mL | 9.3653 mL | 11.7066 mL |
| 10 mM | 0.2341 mL | 1.1707 mL | 2.3413 mL | 4.6826 mL | 5.8533 mL |
| 50 mM | 0.0468 mL | 0.2341 mL | 0.4683 mL | 0.9365 mL | 1.1707 mL |
| 100 mM | 0.0234 mL | 0.1171 mL | 0.2341 mL | 0.4683 mL | 0.5853 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Drim-7-ene-11,12-diol acetonide
Catalog No.:BCN4919
CAS No.:213552-47-7
- Flumethasone
Catalog No.:BCC8986
CAS No.:2135-17-3
- Ercalcidiol
Catalog No.:BCC1555
CAS No.:21343-40-8
- 1-Methyl-L-tryptophan
Catalog No.:BCN8341
CAS No.:21339-55-9
- 18-Nor-4,15-dihydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1494
CAS No.:213329-46-5
- 15,18-Dihydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1495
CAS No.:213329-45-4
- H-Pro-OMe.HCl
Catalog No.:BCC3022
CAS No.:2133-40-6
- RITA (NSC 652287)
Catalog No.:BCC2238
CAS No.:213261-59-7
- [Phe1Ψ(CH2-NH)Gly2]Nociceptin(1-13)NH2
Catalog No.:BCC5701
CAS No.:213130-17-7
- Ceanothic acid
Catalog No.:BCN4918
CAS No.:21302-79-4
- Org 37684
Catalog No.:BCC6291
CAS No.:213007-95-5
- Boc-Tyr(Bzl)-OH
Catalog No.:BCC3461
CAS No.:2130-96-3
- 6,8-Cyclo-1,4-eudesmanediol
Catalog No.:BCN4920
CAS No.:213769-80-3
- Zedoarofuran
Catalog No.:BCN3527
CAS No.:213833-34-2
- Nifenazone
Catalog No.:BCC3822
CAS No.:2139-47-1
- 1-Acetoxy-9,17-octadecadiene-12,14-diyne-11,16-diol
Catalog No.:BCN1493
CAS No.:213905-35-2
- Barbacarpan
Catalog No.:BCN4921
CAS No.:213912-46-0
- Chrysin dimethylether
Catalog No.:BCN6799
CAS No.:21392-57-4
- Taxiphyllin
Catalog No.:BCN4922
CAS No.:21401-21-8
- CART (55-102) (human)
Catalog No.:BCC6007
CAS No.:214050-22-3
- Magnoflorine
Catalog No.:BCN4923
CAS No.:2141-09-5
- Glucoraphanin
Catalog No.:BCN3817
CAS No.:21414-41-5
- 26-Deoxycimicifugoside
Catalog No.:BCN2906
CAS No.:214146-75-5
- 1-Decarboxy-3-oxo-ceanothic acid
Catalog No.:BCN4924
CAS No.:214150-74-0
5-Iodo-A-85380, an alpha4beta2 subtype-selective ligand for nicotinic acetylcholine receptors.[Pubmed:10692507]
Mol Pharmacol. 2000 Mar;57(3):642-9.
In an effort to develop selective radioligands for in vivo imaging of neuronal nicotinic acetylcholine receptors (nAChRs), we synthesized 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine (5-iodo-A-85380) and labeled it with (125)I and (123)I. Here we present the results of experiments characterizing this radioiodinated ligand in vitro. The affinity of 5-[(125)I]iodo-A-85380 for alpha4beta2 nAChRs in rat and human brain is defined by K(d) values of 10 and 12 pM, respectively, similar to that of epibatidine (8 pM). In contrast to epibatidine, however, 5-iodo-A-85380 is more selective in binding to the alpha4beta2 subtype than to other nAChR subtypes. In rat adrenal glands, 5-iodo-A-85380 binds to nAChRs containing alpha3 and beta4 subunits with 1/1000th the affinity of epibatidine, and exhibits 1/60th and 1/190th the affinity of epibatidine at alpha7 and muscle-type nAChRs, respectively. Moreover, unlike epibatidine and cytisine, 5-[(125)I]iodo-A-85380 shows no binding in any brain regions in mice homozygous for a mutation in the beta2 subunit of nAChRs. Binding of 5-[(125)I]iodo-A-85380 in rat brain is reversible, and is characterized by high specificity and a slow rate of dissociation of the receptor-ligand complex (t(1/2) for dissociation approximately 2 h). These properties, along with other features observed previously in in vivo experiments (low toxicity, rapid penetration of the blood-brain barrier, and a high ratio of specific to nonspecific binding), suggest that this compound, labeled with (125)I or (123)I, is superior to other radioligands available for in vitro and in vivo studies of alpha4beta2 nAChRs, respectively.
2-, 5-, and 6-Halo-3-(2(S)-azetidinylmethoxy)pyridines: synthesis, affinity for nicotinic acetylcholine receptors, and molecular modeling.[Pubmed:9733494]
J Med Chem. 1998 Sep 10;41(19):3690-8.
3-(2(S)-Azetidinylmethoxy)pyridine (A-85380) has been identified recently as a ligand with high affinity for nicotinic acetylcholine receptors (nAChRs). Here we report the synthesis and in vitro nAChR binding of a series of 10 pyridine-modified analogues of A-85380. The novel compounds feature a halogen substituent at position 2, 5, or 6 of the 3-pyridyl fragment. Those with the substituents at position 5 or 6, as well as the 2-fluoro analogue, possess subnanomolar affinity for nAChRs in membranes from rat brain. For these ligands, Ki values range from 11 to 210 pM, as measured by competition with (+/-)-[3H]epibatidine. In contrast, 2-chloro, 2-bromo, and 2-iodo analogues exhibit substantially lower affinity. AM1 quantum chemical calculations demonstrate that the bulky substituents at position 2 cause notable changes in the molecular geometry. The high-affinity members of the series and (+)-epibatidine display a tight fit superposition of low-energy stable conformers. The new ligands with high affinity for nAChRs may be of interest as pharmacological probes, potential medications, and candidates for developing radiohalogenated tracers to study nAChRs.


