Dihydroflavokawain CCAS# N/A |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | N/A | SDF | Download SDF |
| PubChem ID | 10266895 | Appearance | Powder |
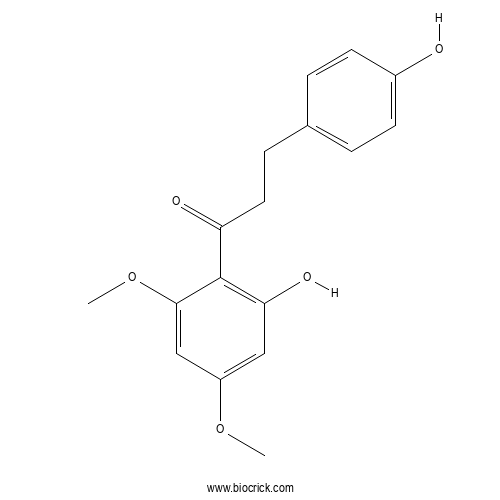
| Formula | C17H18O5 | M.Wt | 302.32 |
| Type of Compound | Chalcones/Dihydrochalcones | Storage | Desiccate at -20°C |
| Synonyms | 4,2'-Dihydroxy-4',6'-dimethoxydihydrochalcone | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(4-hydroxyphenyl)propan-1-one | ||
| SMILES | COC1=CC=C(C=C1)C=CC(=O)C2=C(C=C(C=C2OC)OC)O | ||
| Standard InChIKey | VFHSXDLCCBWTTJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H18O5/c1-21-13-9-15(20)17(16(10-13)22-2)14(19)8-5-11-3-6-12(18)7-4-11/h3-4,6-7,9-10,18,20H,5,8H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Dihydroflavokawain C Dilution Calculator

Dihydroflavokawain C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3078 mL | 16.5388 mL | 33.0775 mL | 66.1551 mL | 82.6938 mL |
| 5 mM | 0.6616 mL | 3.3078 mL | 6.6155 mL | 13.231 mL | 16.5388 mL |
| 10 mM | 0.3308 mL | 1.6539 mL | 3.3078 mL | 6.6155 mL | 8.2694 mL |
| 50 mM | 0.0662 mL | 0.3308 mL | 0.6616 mL | 1.3231 mL | 1.6539 mL |
| 100 mM | 0.0331 mL | 0.1654 mL | 0.3308 mL | 0.6616 mL | 0.8269 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Demethylwedelolactone
Catalog No.:BCN2663
CAS No.:6468-55-9
- Coleonol B
Catalog No.:BCN4188
CAS No.:64657-21-2
- 1,9-Dideoxyforskolin
Catalog No.:BCC6352
CAS No.:64657-18-7
- Demethylluvangetin
Catalog No.:BCN7569
CAS No.:64652-10-4
- VcMMAE
Catalog No.:BCC2033
CAS No.:646502-53-6
- H-D-Glu(OMe)-OH
Catalog No.:BCC2940
CAS No.:6461-04-7
- Sinigrin monohydrate
Catalog No.:BCN2595
CAS No.:64550-88-5
- Acantrifoside E
Catalog No.:BCN6646
CAS No.:645414-25-1
- Oxprenolol hydrochloride
Catalog No.:BCC7630
CAS No.:6452-73-9
- Scoulerine
Catalog No.:BCN6623
CAS No.:6451-73-6
- Isovanillic acid
Catalog No.:BCN3376
CAS No.:645-08-9
- Altretamine
Catalog No.:BCC1216
CAS No.:645-05-6
- Cinaciguat hydrochloride
Catalog No.:BCC8096
CAS No.:646995-35-9
- Morin dihydrate
Catalog No.:BCN8156
CAS No.:6472-38-4
- RA-V
Catalog No.:BCN3513
CAS No.:64725-24-2
- Tetrahydroalstonine
Catalog No.:BCN4189
CAS No.:6474-90-4
- 1,2,3,6,7-Pentamethoxyxanthone
Catalog No.:BCN7525
CAS No.:64756-86-1
- 6-Hydroxy-1,2,3,7-tetramethoxyxanthone
Catalog No.:BCN7565
CAS No.:64756-87-2
- L-trans-2,4-PDC
Catalog No.:BCC6595
CAS No.:64769-66-0
- Betulalbuside A
Catalog No.:BCN4190
CAS No.:64776-96-1
- Trilobatin 2''-acetate
Catalog No.:BCN4191
CAS No.:647853-82-5
- Glucagon (19-29), human
Catalog No.:BCC1012
CAS No.:64790-15-4
- Heraclenol acetonide
Catalog No.:BCN4192
CAS No.:64790-68-7
- Neomangiferin
Catalog No.:BCN4970
CAS No.:64809-67-2
Induction of G2M Arrest by Flavokawain A, a Kava Chalcone, Increases the Responsiveness of HER2-Overexpressing Breast Cancer Cells to Herceptin.[Pubmed:28335434]
Molecules. 2017 Mar 14;22(3). pii: molecules22030462.
HER2/neu positive breast tumors predict a high mortality and comprise 25%-30% of breast cancer. We have shown that Flavokawain A (FKA) preferentially reduces the viabilities of HER2-overexpressing breast cancer cell lines (i.e., SKBR3 and MCF7/HER2) versus those with less HER2 expression (i.e., MCF7 and MDA-MB-468). FKA at cytotoxic concentrations to breast cancer cell lines also has a minimal effect on the growth of non-malignant breast epithelial MCF10A cells. FKA induces G2M arrest in cell cycle progression of HER2-overexpressing breast cancer cell lines through inhibition of Cdc2 and Cdc25C phosphorylation and downregulation of expression of Myt1 and Wee1 leading to increased Cdc2 kinase activities. In addition, FKA induces apoptosis in SKBR3 cells by increasing the protein expression of Bim and BAX and decreasing expression of Bcl(2), BclX/L, XIAP, and survivin. FKA also downregulates the protein expression of HER-2 and inhibits AKT phosphorylation. Herceptin plus FKA treatment leads to an enhanced growth inhibitory effect on HER-2 overexpressing breast cancer cell lines through downregulation of Myt1, Wee1, Skp2, survivin, and XIAP. Our results suggest FKA as a promising and novel apoptosis inducer and G2 blocking agent that, in combination with Herceptin, enhances for the treatment of HER2-overexpressing breast cancer.
Flavokawain A inhibits Cytochrome P450 in in vitro metabolic and inhibitory investigations.[Pubmed:27318274]
J Ethnopharmacol. 2016 Sep 15;191:350-359.
ETHNOPHARMACOLOGICAL RELEVANCE: Flavokawain A, the major chalcone in kava extracts, was served as beverages for informal social occasions and traditional ceremonials in most South Pacific islands. It exhibited strong antiproliferative and apoptotic effects against human prostate and urinary bladder cancer cells. AIM OF THE STUDY: The current study was purposed to investigate the interaction between Flavokawain A and Cytochrome P450, including the inhibitory effects of Flavokawain A on predominant CYP450 isotypes and further clarified the inhibitory mechanism of FKA on CYP450 enzymes. Besides, study about identifying the key CYP450 isotypes responsible for the metabolism of FKA was also performed. MATERIALS AND METHODS: In this study, probe-based assays with rat liver microsome system were used to characterize the inhibitory effects of FKA. Molecular docking study was performed to further explore the binding site of FKA on CYP450 isoforms. In addition, chemical inhibition experiments using specific inhibitors (a-naphthoflavone, quinidine, sulfamethoxazde, ketoconazole, omeprazole) were performed to clarify the individual CYP450 isoform that are responsible for the metabolism of FKA. RESULTS: FKA showed significant inhibition on CYP1A2, CYP2D1, CYP2C6 and CYP3A2 activities with IC50 values of 102.23, 20.39, 69.95, 60.22mumol/L, respectively. The inhibition model was competitive, mixed-inhibition, uncompetitive, and noncompetitive for CYP1A2, CYP2D1, CYP2C6 and CYP3A2 enzymes. Molecular docking study indicated the ligand-binding conformation of FKA in the active site of CYP450 isoforms. The chemical inhibition experiments showed that the metabolic clearance rate of Flavokawain A decreased to 19.84%, 50.38%, and 67.02% of the control in the presence of ketoconazole, sulfamethoxazde and a-naphthoflavone. CONCLUSION: The study showed that Flavokawain A has varying inhibitory effect on CYP450 enzymes and CYP3A2 was the principal CYP isoform contributing to the metabolism of Flavokawain A. Besides, CYP2C6 and CYP1A2 isoforms also play important roles in the metabolism of FKA. Our results provided a basis for better understanding the biotransformation of FKA and prediction of drug-drug interaction of FKA.
Flavokawain A induces deNEDDylation and Skp2 degradation leading to inhibition of tumorigenesis and cancer progression in the TRAMP transgenic mouse model.[Pubmed:26497688]
Oncotarget. 2015 Dec 8;6(39):41809-24.
S phase kinase-associated protein 2 (Skp2) has been shown to be required for spontaneous tumor development that occurs in the retinoblastoma protein (pRb) deficient mice. Here we have demonstrated that flavokawain A (FKA), a novel chalcone from the kava plant, selectively inhibited the growth of pRb deficient cell lines and resulted in a proteasome-dependent and ubiquitination-mediated Skp2 degradation. Degradation of Skp2 by FKA was found to be involved in a functional Cullin1, but independent of Cdh1 expression. Further studies have demonstrated that FKA docked into the ATP binding pocket of the precursor cell-expressed developmentally down-regulated 8 (NEDD8)-activating enzyme (NAE) complex, inhibited NEDD8 conjugations to both Cullin1 and Ubc12 in PC3 cells and Ubc12 NEDDylation in an in vitro assay. Finally, dietary feeding of the autochthonous transgenic adenocarcinoma of the mouse prostate (TRAMP) mice with FKA inhibited the formation of high-grade prostatic intra-epithelial neoplasia lesions (HG-PIN) and prostate adenocarcinomas, reduced the tumor burden and completely abolished distant organ metastasis. Immunohistochemistry studies revealed that dietary FKA feeding resulted in marked anti-proliferative and apoptotic effects via down-regulation of Skp2 and NEDD8 and up-regulation of p27/Kip1 in the prostate of TRAMP mice. Our findings therefore provide evidence that FKA is a promising NEDDylation inhibitor for targeting Skp2 degradation in prostate cancer prevention and treatment.
In vitro Toxicity and in vivo Immunomodulatory Effects of Flavokawain A and Flavokawain B in Balb/C Mice.[Pubmed:26411010]
Nat Prod Commun. 2015 Jul;10(7):1199-202.
Flavokawains are chalcones that can be found in the root extracts of the kava-kava (Piper methysticum) plant. Flavokawain A and flavokawain B are known to possess potential anti-inflammation and anti-cancer activities. Nevertheless, the effects of both these compounds on the normal function of the host have not been studied. There is a need to find agents that can enhance the functionality of the immune system without disturbing the homeostatic balance. This study aimed to determine the toxicity and immunomodulatory effects of flavokawain A and flavokawain B on Balb/c mice. Several assays were conducted, the MTT viability assay, cytokine detection (IL-2 and TNF-alpha), immunophenotyping of important immune markers, serum biochemical analysis and detection of nitric oxide levels. Based on our results, flavokawain A and B did not cause mortality and all mice were observed normal after the treatment period. Both flavokawains stimulated splenocyte proliferation, the secretion of IL-2 and TNF-alpha and raised the population of T cell subsets without significantly altering the level of several serum biochemical parameters. Overall, flavokawain A and B could serve as potential immune-modulator drugs without causing any toxicity, however further in vivo evidence is needed.
In Vivo Anti-Tumor Effects of Flavokawain A in 4T1 Breast Cancer Cell-Challenged Mice.[Pubmed:26179368]
Anticancer Agents Med Chem. 2015;15(7):905-15.
Flavokawain A is a chalcone that can be found in the kava-kava plant (Piper methsyticum) extract. The kava-kava plant has been reported to possess anti-cancer, anti-inflammatory and antinociceptive activities. The state of the immune system, and the inflammatory process play vital roles in the progression of cancer. The immunomodulatary effects and the anti-inflammatory effects of flavokawain A in a breast cancer murine model have not been studied yet. Thus, this study aimed to elucidate the basic mechanism as to how flavokawain A regulates and enhance the immune system as well as impeding the inflammatory process in breast cancer-challenged mice. Based on our study, it is interesting to note that flavokawain A increased the T cell population; both Th1 cells and CTLs, aside from the natural killer cells. The levels of IFN-gamma and IL-2 were also elevated in the serum of flavokawain A-treated mice. Apart from that, flavokawain A also decreased the weight and volume of the tumor, and managed to induce apoptosis in them. In terms of inflammation, flavokawain A-treated mice had reduced level of major pro-inflammatory mediators; NO, iNOS, NF-KB, ICAM and COX-2. Overall, flavokawain A has the potential to not only enhance antitumor immunity, but also prevents the inflammatory process in a cancer-prone microenvironment.
Dietary feeding of Flavokawain A, a Kava chalcone, exhibits a satisfactory safety profile and its association with enhancement of phase II enzymes in mice.[Pubmed:25379458]
Toxicol Rep. 2014;1:2-11.
Flavokawain A (FKA), a major chalcone in the Kava plant, has recently demonstrated promising anti-cancer activities. A systematic evaluation of FKA's safety profile has not been reported before. In this study, male FVB/N mice were fed with an AIN-76A diet or AIN-76A diet supplemented with 0.6% (6 g/kg food) FKA or 0.6% commercial kava root extract (KRE) for three weeks. Dietary feeding of FKA did not affect food consumption and body weight. Histopathological examination of liver, kidney, colon, lung, heart, spleen, and thymus revealed no signs of FKA-induced toxicity. Biochemical serum analysis and histological examination confirmed normal organ function in FKA-treated mice. The cytotoxicity profile showed FKA had minimal side effects on bone marrow and small intestinal epithelial cells compared with Adriamycin. In addition, oral feeding of FKA increased activities of both glutathione S-transferase and quinone reductase in the liver, lung, prostate and bladder tissues of mice. In comparison, dietary feeding of 0.6% KRE increased liver/body weight ratio and decreased spleen, thymus, and testis/body weight ratios, as well as induced nodular proliferation in liver tissues. Therefore, dietary feeding FKA showed no adverse effects on major organ function and homeostasis in mice, suggesting the potential of FKA for chemoprevention study of human cancers.


