FebrifugineCAS# 24159-07-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 24159-07-7 | SDF | Download SDF |
| PubChem ID | 63224 | Appearance | Powder |
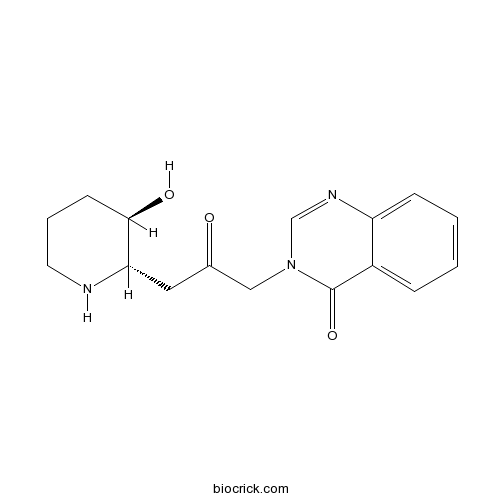
| Formula | C16H19N3O3 | M.Wt | 301.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-[3-[(2S,3R)-3-hydroxypiperidin-2-yl]-2-oxopropyl]quinazolin-4-one | ||
| SMILES | C1CC(C(NC1)CC(=O)CN2C=NC3=CC=CC=C3C2=O)O | ||
| Standard InChIKey | FWVHWDSCPKXMDB-LSDHHAIUSA-N | ||
| Standard InChI | InChI=1S/C16H19N3O3/c20-11(8-14-15(21)6-3-7-17-14)9-19-10-18-13-5-2-1-4-12(13)16(19)22/h1-2,4-5,10,14-15,17,21H,3,6-9H2/t14-,15+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Febrifugine is an effective coccidiostat, possesses schizonticide props; it and its derivatives shows high degree of antimalarial activity but use limited by toxicity . |
| Targets | Antifection | PfcPRS | IL Receptor | IFN-γ |
| In vivo | The cytoplasmic prolyl-tRNA synthetase of the malaria parasite is a dual-stage target of febrifugine and its analogs.[Pubmed: 25995223]Sci Transl Med. 2015 May 20;7(288):288ra77.The emergence of drug resistance is a major limitation of current antimalarials. The discovery of new druggable targets and pathways including those that are critical for multiple life cycle stages of the malaria parasite is a major goal for developing next-generation antimalarial drugs. Possible involvement of IFN-gamma in early mortality of Plasmodium berghei NK65-infected BALB/c mice after febrifugine treatment.[Pubmed: 19062681]Southeast Asian J Trop Med Public Health. 2008 Nov;39(6):949-58.
|
| Structure Identification | J Adv Pharm Technol Res. 2013 Jan;4(1):50-60.Pharmacophore modeling and 3D quantitative structure-activity relationship analysis of febrifugine analogues as potent antimalarial agent.[Pubmed: 23662282]Febrifugine and its derivatives are effective against Plasmodium falciparum. |

Febrifugine Dilution Calculator

Febrifugine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.319 mL | 16.5948 mL | 33.1895 mL | 66.379 mL | 82.9738 mL |
| 5 mM | 0.6638 mL | 3.319 mL | 6.6379 mL | 13.2758 mL | 16.5948 mL |
| 10 mM | 0.3319 mL | 1.6595 mL | 3.319 mL | 6.6379 mL | 8.2974 mL |
| 50 mM | 0.0664 mL | 0.3319 mL | 0.6638 mL | 1.3276 mL | 1.6595 mL |
| 100 mM | 0.0332 mL | 0.1659 mL | 0.3319 mL | 0.6638 mL | 0.8297 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-(4-Methylphenyl)-3-oxobutanamide
Catalog No.:BCC9058
CAS No.:2415-85-2
- Catalpol
Catalog No.:BCN5094
CAS No.:2415-24-9
- Isavuconazole
Catalog No.:BCC5515
CAS No.:241479-67-4
- TMS
Catalog No.:BCC7093
CAS No.:24144-92-1
- Khellactone
Catalog No.:BCN6684
CAS No.:24144-61-4
- 2-CMDO
Catalog No.:BCC5671
CAS No.:24140-98-5
- Flupenthixol dihydrochloride
Catalog No.:BCC7851
CAS No.:2413-38-9
- Adaphostin
Catalog No.:BCC3890
CAS No.:241127-58-2
- Sibiricaxanthone B
Catalog No.:BCN2784
CAS No.:241125-81-5
- Sibiricose A6
Catalog No.:BCN2786
CAS No.:241125-75-7
- 6-Isopentenyloxyisobergapten
Catalog No.:BCC8110
CAS No.:24099-29-4
- Digiferruginol
Catalog No.:BCN3450
CAS No.:24094-45-9
- Trametenolic acid
Catalog No.:BCN3330
CAS No.:24160-36-9
- Epitulipinolide
Catalog No.:BCN5095
CAS No.:24164-13-4
- Kobusone
Catalog No.:BCN5096
CAS No.:24173-71-5
- Zoniporide dihydrochloride
Catalog No.:BCC7461
CAS No.:241799-10-0
- H-Lys(Boc)-OH
Catalog No.:BCC2982
CAS No.:2418-95-3
- H-Glu(OtBu)-OH
Catalog No.:BCC2933
CAS No.:2419-56-9
- Boc-Glu-OH
Catalog No.:BCC3386
CAS No.:2419-94-5
- Epiyangambin
Catalog No.:BCN7029
CAS No.:24192-64-1
- Bis(4-hydroxy-3-methylphenyl) sulfide
Catalog No.:BCC8885
CAS No.:24197-34-0
- 3,3',4,4'-Benzophenone tetracarboxylic dianhydride
Catalog No.:BCC8593
CAS No.:2421-28-5
- Farrerol
Catalog No.:BCN2481
CAS No.:24211-30-1
- Tabimorelin hemifumarate
Catalog No.:BCC5897
CAS No.:242143-80-2
Possible involvement of IFN-gamma in early mortality of Plasmodium berghei NK65-infected BALB/c mice after febrifugine treatment.[Pubmed:19062681]
Southeast Asian J Trop Med Public Health. 2008 Nov;39(6):949-58.
Parasitemia patterns, survival and cytokine levels of Plasmodium berghei NK65-infected BALB/c mice, treated orally with the alkaloidal mixture of Febrifugine and isoFebrifugine at a dose of 1 mg/kg twice a day for 4 consecutive days were monitored. Whereas the untreated mice showed a progressive increase in parasitemia and ultimate death, the alkaloid mixture-treated group showed a transient suppression of parasitemia during the course of treatment. However, the parasitemia increased on discontinuation of treatment, leading to earlier death of mice in the treated group than in the infected but untreated controls. Mice in the infected but untreated group displayed a significant elevation in serum IFN-gammay levels during the first week post-infection (pI) and from Day 14 pI, relative to the levels in the uninfected controls. In contrast, although mice in the alkaloid mixture-treated group displayed no significant elevation in serum IFN-gamma levels during the first week pI, they showed considerable levels on Day 14 pI. There were no significant differences in serum IL-4 levels among the groups. The titers of the parasite-specific IgG1, IgG2a, IgG2b and IgG3 were significantly elevated from Day 11 pI in both the treated and untreated groups. There was a significant difference in survival duration between the IFN-gamma-/- mutant and BALB/c mice. IFN-gamma-/- mutant mice showed a decrease in parasitemia levels while receiving medication, which was significantly lower than those of the treated BALB/c mice. The results of the present study suggest that although IFN-gamma is significant for protective immunity in mice with malaria infection, it may play an adverse role post-medication, causing earlier mortality of treated BALB/c mice.
The cytoplasmic prolyl-tRNA synthetase of the malaria parasite is a dual-stage target of febrifugine and its analogs.[Pubmed:25995223]
Sci Transl Med. 2015 May 20;7(288):288ra77.
The emergence of drug resistance is a major limitation of current antimalarials. The discovery of new druggable targets and pathways including those that are critical for multiple life cycle stages of the malaria parasite is a major goal for developing next-generation antimalarial drugs. Using an integrated chemogenomics approach that combined drug resistance selection, whole-genome sequencing, and an orthogonal yeast model, we demonstrate that the cytoplasmic prolyl-tRNA (transfer RNA) synthetase (PfcPRS) of the malaria parasite Plasmodium falciparum is a biochemical and functional target of Febrifugine and its synthetic derivative halofuginone. Febrifugine is the active principle of a traditional Chinese herbal remedy for malaria. We show that treatment with Febrifugine derivatives activated the amino acid starvation response in both P. falciparum and a transgenic yeast strain expressing PfcPRS. We further demonstrate in the Plasmodium berghei mouse model of malaria that halofuginol, a new halofuginone analog that we developed, is active against both liver and asexual blood stages of the malaria parasite. Halofuginol, unlike halofuginone and Febrifugine, is well tolerated at efficacious doses and represents a promising lead for the development of dual-stage next-generation antimalarials.
Pharmacophore modeling and 3D quantitative structure-activity relationship analysis of febrifugine analogues as potent antimalarial agent.[Pubmed:23662282]
J Adv Pharm Technol Res. 2013 Jan;4(1):50-60.
Febrifugine and its derivatives are effective against Plasmodium falciparum. Using PHASE algorithm, a five-point pharmacophore model with two hydrogen bond acceptor (A), one positively ionizable (P) and two aromatic rings (R), was developed to derive a predictive ligand-based statistically significant 3D-quantitative structure-activity relationship (QSAR) model (r(2) = 0.972, SD = 0.3, F = 173.4, Q(2) = 0.712, RMSE = 0.3, Person-R = 0.94, and r(2) pred = 0.8) to explicate the structural attributes crucial for antimalarial activity. The developed pharmacophore model and 3D QSAR model can be a substantial tool for virtual screening and related antimalarial drug discovery research.


