H-Lys(Boc)-OHCAS# 2418-95-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2418-95-3 | SDF | Download SDF |
| PubChem ID | 2733283 | Appearance | Powder |
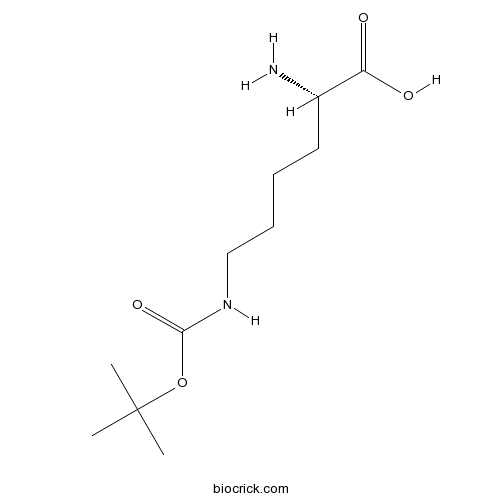
| Formula | C11H22N2O4 | M.Wt | 246.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | (2S)-2-amino-6-[(2-methylpropan-2-yl)oxycarbonylamino]hexanoic acid | ||
| SMILES | CC(C)(C)OC(=O)NCCCCC(C(=O)O)N | ||
| Standard InChIKey | VVQIIIAZJXTLRE-QMMMGPOBSA-N | ||
| Standard InChI | InChI=1S/C11H22N2O4/c1-11(2,3)17-10(16)13-7-5-4-6-8(12)9(14)15/h8H,4-7,12H2,1-3H3,(H,13,16)(H,14,15)/t8-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

H-Lys(Boc)-OH Dilution Calculator

H-Lys(Boc)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0601 mL | 20.3004 mL | 40.6009 mL | 81.2018 mL | 101.5022 mL |
| 5 mM | 0.812 mL | 4.0601 mL | 8.1202 mL | 16.2404 mL | 20.3004 mL |
| 10 mM | 0.406 mL | 2.03 mL | 4.0601 mL | 8.1202 mL | 10.1502 mL |
| 50 mM | 0.0812 mL | 0.406 mL | 0.812 mL | 1.624 mL | 2.03 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.406 mL | 0.812 mL | 1.015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Lys(Boc)-OH
- Zoniporide dihydrochloride
Catalog No.:BCC7461
CAS No.:241799-10-0
- Kobusone
Catalog No.:BCN5096
CAS No.:24173-71-5
- Epitulipinolide
Catalog No.:BCN5095
CAS No.:24164-13-4
- Trametenolic acid
Catalog No.:BCN3330
CAS No.:24160-36-9
- Febrifugine
Catalog No.:BCN3269
CAS No.:24159-07-7
- N-(4-Methylphenyl)-3-oxobutanamide
Catalog No.:BCC9058
CAS No.:2415-85-2
- Catalpol
Catalog No.:BCN5094
CAS No.:2415-24-9
- Isavuconazole
Catalog No.:BCC5515
CAS No.:241479-67-4
- TMS
Catalog No.:BCC7093
CAS No.:24144-92-1
- Khellactone
Catalog No.:BCN6684
CAS No.:24144-61-4
- 2-CMDO
Catalog No.:BCC5671
CAS No.:24140-98-5
- Flupenthixol dihydrochloride
Catalog No.:BCC7851
CAS No.:2413-38-9
- H-Glu(OtBu)-OH
Catalog No.:BCC2933
CAS No.:2419-56-9
- Boc-Glu-OH
Catalog No.:BCC3386
CAS No.:2419-94-5
- Epiyangambin
Catalog No.:BCN7029
CAS No.:24192-64-1
- Bis(4-hydroxy-3-methylphenyl) sulfide
Catalog No.:BCC8885
CAS No.:24197-34-0
- 3,3',4,4'-Benzophenone tetracarboxylic dianhydride
Catalog No.:BCC8593
CAS No.:2421-28-5
- Farrerol
Catalog No.:BCN2481
CAS No.:24211-30-1
- Tabimorelin hemifumarate
Catalog No.:BCC5897
CAS No.:242143-80-2
- Mianserin
Catalog No.:BCC4134
CAS No.:24219-97-4
- 3-Methyl-9H-carbazol-2-ol
Catalog No.:BCN4666
CAS No.:24224-30-4
- Thalictrimine
Catalog No.:BCN5097
CAS No.:24240-04-8
- Solifenacin succinate
Catalog No.:BCC4580
CAS No.:242478-38-2
- 2,5-Bis(4-aminophenyl)-1,3,4-oxadiazole
Catalog No.:BCC8501
CAS No.:2425-95-8
Synthesis and functional studies of tuftsin analogs containing isopeptide bond.[Pubmed:2381868]
Peptides. 1990 May-Jun;11(3):405-15.
In the present paper a new approach is reported, to increase the resistance of tuftsin toward enzymatic cleavage by the introduction of an isopeptide bond into the molecule. The tetrapeptides H-Lys(Thr)-Pro-Arg-OH and H-Lys(Ala)-Pro-Arg-OH, the pentapeptides H-Thr-Lys(Ala)-Pro-Arg-OH, H-Thr-Lys(Thr)-Pro-Arg-OH and H-Ala-Lys(Ala)-Pro-Arg-OH and their For- and Boc-protected derivatives were built up by stepwise elongation of the chain, using conventional solution-phase methods. Preliminary experiments confirmed that from the Lys residue in position 2 of tuftsin the alpha-peptide bond between the Thr and Lys is cleaved with a significantly higher rate by leucine aminopeptidase than the epsilon-peptide bond. Several of the isopeptide derivatives increased to a higher extent the interleukin (IL-1) secretion by monocytes than tuftsin or [Ala1]-tuftsin.
Surfaces having dual fibrinolytic and protein resistant properties by immobilization of lysine on polyurethane through a PEG spacer.[Pubmed:18646203]
J Biomed Mater Res A. 2009 Sep 1;90(3):940-6.
The objective of this work is to develop a blood contacting surface that possesses both resistance to nonspecific protein adsorption and clot lysing properties. Chemical modification of a polyurethane (PU) surface with poly(ethylene glycol) (PEG); and lysine was used to create a plasminogen-binding potentially fibrinolytic surface. The preparation involves modification of the PU surface with dihydroxy PEG, reaction of the unreacted distal OH with N,N'-disuccinimidyl carbonate (DSC) to produce a PU-PEG-NHS surface, followed by conjugation of epsilon-amino-protected lysine (H-Lys(t-BOC)-OH) by reaction of the alpha-amino group with the NHS and deprotection. The result is a lysine-derivatized surface in which the epsilon-amino groups of the lysine are free to participate in binding plasminogen and tissue plasminogen activator (t-PA). Surfaces were characterized by X-ray photoelectron spectroscopy (XPS) and contact angle measurements. Protein adsorption experiments showed that nonspecific protein adsorption was greatly reduced on these surfaces and that they adsorbed significant quantities of plasminogen from plasma. After incubation with plasma and treatment with t-PA the surfaces were able to dissolve nascent plasma clots formed around them.
Synthesis of modified tuftsins containing monosaccharides or monosaccharide derivatives.[Pubmed:3570665]
Int J Pept Protein Res. 1987 Feb;29(2):250-61.
Synthesis of some modified tuftsins is described in which a monosaccharide or a monosaccharide derivative was incorporated in the molecule. Acylation of H-Thr-Lys(Z)-Pro-Arg(NO2)-OBzl with D(+)-gluco-1,5-lactone followed by catalytic hydrogenation gave N alpha-gluconyl-tuftsin. Glycosylation of the carboxyl function of the C-terminal arginine has been achieved by reacting, through the mixed anhydride procedure, Boc-Thr-Lys(Z)-Pro-OH with 2-deoxy-2-(NG-nitroargininamido)-D-glucopyranose followed by catalytic hydrogenation and trifluoroacetic acid treatment. O-Glucosyl-tuftsin has been prepared by reacting o-nitrophenyl N-benzyloxycarbonyl-O-[(alpha + beta) 2,3,4,6-tetra-O-benzyl-D-glucopyranosyl]-threoninate with H-Lys(Z)-Pro-Arg(NO2)-OBzl in the presence of 1-hydroxybenzotriazole. Flash chromatography on silica gel allowed a partial separation of the diastereoisomers, one of which has been isolated in a reasonable yield. The single diastereoisomer and the alpha + beta anomeric mixture were separately deblocked by catalytic hydrogenation and purified by RP-HPLC.
Synthesis, pharmacokinetics, and biological use of lysine-modified single-walled carbon nanotubes.[Pubmed:25228803]
Int J Nanomedicine. 2014 Sep 4;9:4245-55.
We aimed to create a more robust and more accessible standard for amine-modifying single-walled carbon nanotubes (SWCNTs). A 1,3-cycloaddition was developed using an azomethine ylide, generated by reacting paraformaldehyde and a side-chain-Boc (tert-Butyloxycarbonyl)-protected, lysine-derived alpha-amino acid, H-Lys(Boc)-OH, with purified SWCNT or C60. This cycloaddition and its lysine adduct provides the benefits of dense, covalent modification, ease of purification, commercial availability of reagents, and pH-dependent solubility of the product. Subsequently, SWCNTs functionalized with lysine amine handles were covalently conjugated to a radiometalated chelator, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA). The (111)In-labeled construct showed rapid renal clearance in a murine model and a favorable biodistribution, permitting utility in biomedical applications. Functionalized SWCNTs strongly wrapped small interfering RNA (siRNA). In the first disclosed deployment of thermophoresis with carbon nanotubes, the lysine-modified tubes showed a desirable, weak SWCNT-albumin binding constant. Thus, lysine-modified nanotubes are a favorable candidate for medicinal work.


