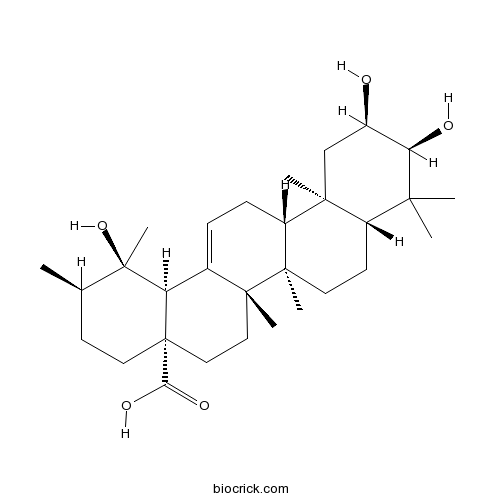
Euscaphic acidCAS# 53155-25-2 |
- 2-Epitormentic acid
Catalog No.:BCN6084
CAS No.:119725-19-8
- Tormentic acid
Catalog No.:BCN6198
CAS No.:13850-16-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 53155-25-2 | SDF | Download SDF |
| PubChem ID | 471426 | Appearance | Powder |
| Formula | C30H48O5 | M.Wt | 488.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2R,4aS,6aR,6aS,6bR,8aR,10S,11R,12aR,14bS)-1,10,11-trihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | ||
| SMILES | CC1CCC2(CCC3(C(=CCC4C3(CCC5C4(CC(C(C5(C)C)O)O)C)C)C2C1(C)O)C)C(=O)O | ||
| Standard InChIKey | OXVUXGFZHDKYLS-QUFHAEKXSA-N | ||
| Standard InChI | InChI=1S/C30H48O5/c1-17-10-13-30(24(33)34)15-14-27(5)18(22(30)29(17,7)35)8-9-21-26(4)16-19(31)23(32)25(2,3)20(26)11-12-28(21,27)6/h8,17,19-23,31-32,35H,9-16H2,1-7H3,(H,33,34)/t17-,19-,20+,21-,22-,23-,26+,27-,28-,29-,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Euscaphic acid has anti-diabetic activity. 2. Euscaphic acid induces death by activation of caspase-3, dependent apoptotic pathway. 3. Euscaphic acid and tormentic acid have inhibitory effect on high fat diet-induced obesity in the rat. 4. Euscaphic acid has anti-contraction effects on rat’s aortic smooth muscle. 5. Euscaphic acid has anti-inflammatory activity, inhibits LPS-induced inflammatory responses by interference with the clustering of TRAF6 with IRAK1 and TAK1, resulting in blocking the activation of IKK and MAPKs signal transduction to downregulate NF-κB activations. |
| Targets | LDL | NO | PGE | TNF-α | NOS | COX | NF-kB | JNK | ERK | p38MAPK | Calcium Channel | Sodium Channel | ATPase | Potassium Channel | IkB | IKK |

Euscaphic acid Dilution Calculator

Euscaphic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0462 mL | 10.2312 mL | 20.4625 mL | 40.9249 mL | 51.1561 mL |
| 5 mM | 0.4092 mL | 2.0462 mL | 4.0925 mL | 8.185 mL | 10.2312 mL |
| 10 mM | 0.2046 mL | 1.0231 mL | 2.0462 mL | 4.0925 mL | 5.1156 mL |
| 50 mM | 0.0409 mL | 0.2046 mL | 0.4092 mL | 0.8185 mL | 1.0231 mL |
| 100 mM | 0.0205 mL | 0.1023 mL | 0.2046 mL | 0.4092 mL | 0.5116 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Buprenorphine hydrochloride
Catalog No.:BCC5215
CAS No.:53152-21-9
- Rapamycin (Sirolimus)
Catalog No.:BCC3592
CAS No.:53123-88-9
- Boc-Pyr-OH
Catalog No.:BCC3329
CAS No.:53100-44-0
- 4',7-Isoflavandiol
Catalog No.:BCN2855
CAS No.:531-95-3
- Coumarin-3-Carboxylic Acid
Catalog No.:BCC9220
CAS No.:531-81-7
- Esculin
Catalog No.:BCN5904
CAS No.:531-75-9
- 7-Methoxycoumarin
Catalog No.:BCN2707
CAS No.:531-59-9
- Scopolin
Catalog No.:BCN5701
CAS No.:531-44-2
- Coniferin
Catalog No.:BCN5700
CAS No.:531-29-3
- Androsin
Catalog No.:BCN3842
CAS No.:531-28-2
- Dichotomin
Catalog No.:BCN2836
CAS No.:53093-47-3
- 9,13-Epidioxy-8(14)-abieten-18-oic acid
Catalog No.:BCN1426
CAS No.:5309-35-3
- Delphinidin-3-sambubioside chloride
Catalog No.:BCN3148
CAS No.:53158-73-9
- Acemetacin
Catalog No.:BCC4424
CAS No.:53164-05-9
- Pirfenidone
Catalog No.:BCC5086
CAS No.:53179-13-8
- 6-Aminoindole
Catalog No.:BCC8763
CAS No.:5318-27-4
- Fagomine
Catalog No.:BCC1569
CAS No.:53185-12-9
- Etomidate hydrochloride
Catalog No.:BCC4255
CAS No.:53188-20-8
- Methyocarbamol
Catalog No.:BCC3813
CAS No.:532-03-6
- Anethole trithione
Catalog No.:BCN8510
CAS No.:532-11-6
- Tropinone
Catalog No.:BCN1935
CAS No.:532-24-1
- Euparin
Catalog No.:BCN7191
CAS No.:532-48-9
- ar-Turmerone
Catalog No.:BCN7516
CAS No.:532-65-0
- Coixol
Catalog No.:BCN5703
CAS No.:532-91-2
Euscaphic acid isolated from roots of Rosa rugosa inhibits LPS-induced inflammatory responses via TLR4-mediated NF-kappaB inactivation in RAW 264.7 macrophages.[Pubmed:22234926]
J Cell Biochem. 2012 Jun;113(6):1936-46.
As an attempt to search for bioactive natural products exerting anti-inflammatory activity, we have evaluated the anti-inflammatory effects of Euscaphic acid (19alpha-hydroxyursane-type triterpenoids, EA) isolated from roots of Rosa rugosa and its underlying molecular mechanisms in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages. EA concentration-dependently reduced the production of nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor-alpha (TNF-alpha), and interleukin-1beta (IL-1beta) induced by LPS in RAW 264.7 macgophages. Consistent with these data, expression levels of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) protein and iNOS, COX-2, TNF-alpha, and IL-1beta mRNA were inhibited by EA in a concentration-dependent manner. In addition, EA attenuated LPS-induced DNA binding and transcriptional activity of nuclear factor-kappa B (NF-kappaB), which was accompanied by a parallel reduction of degradation and phosphorylation of inhibitory kappa Balpha (IkappaBalpha) and consequently by decreased nuclear translocation of p65 subunit of NF-kappaB. Pretreatment with EA significantly inhibited the LPS-induced phosphorylation of IkappaB kinase beta (IKKbeta), p38, and JNK, whereas the phosphorylation of ERK1/2 was unaffected. Furthermore, EA interfered with the LPS-induced clustering of TNF receptor-associated factor 6 (TRAF6) with interleukin receptor associated kinase 1 (IRAK1) and transforming growth factor-beta-activated kinase 1 (TAK1). Taken together, these results suggest that EA inhibits LPS-induced inflammatory responses by interference with the clustering of TRAF6 with IRAK1 and TAK1, resulting in blocking the activation of IKK and MAPKs signal transduction to downregulate NF-kappaB activations.
Euscaphic acid, a new hypoglycemic natural product from Folium Eriobotryae.[Pubmed:18972842]
Pharmazie. 2008 Oct;63(10):765-7.
Folium Eriobotryae has been used as a medicinal plant for a long time, and it is known to have many physiological actions such as anti-inflammatory, anti-tussive, expectorant and anti-diabetic. We have reported that the 70% ethanol extract of Folium Eriobotryae exerted a significant hypoglycemic effect to alloxan-diabetic mice. In this study, we isolated Euscaphic acid, a natural product from Folium Eriobotryae, and investigated its hypoglycemic effect in normoglycemic and alloxan-diabetic mice. All effects had been compared with those of gliclazide. The plasma glucose levels were significantly lowered in normoglycemic mice treated with Euscaphic acid compared to mice treated with 0.5% CMC-Na solution only. Moreover, the dosage of 50 mg/kg exerted a significant (P < 0.05) hypoglycemic effect in alloxan-diabetic mice after orally administration. The research proved that Euscaphic acid is one of the active hypoglycemic constituents in Folium Eriobotryae, but the details of the mechanism need to be investigated further.


