DichotominCAS# 53093-47-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53093-47-3 | SDF | Download SDF |
| PubChem ID | 3085030 | Appearance | Powder |
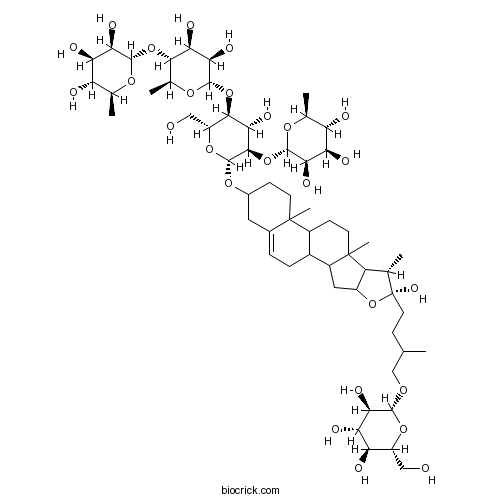
| Formula | C57H94O26 | M.Wt | 1195.34 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1C2C(CC3C2(CCC4C3CC=C5C4(CCC(C5)OC6C(C(C(C(O6)CO)OC7C(C(C(C(O7)C)OC8C(C(C(C(O8)C)O)O)O)O)O)O)OC9C(C(C(C(O9)C)O)O)O)C)C)OC1(CCC(C)COC1C(C(C(C(O1)CO)O)O)O)O | ||
| Standard InChIKey | YHKROSUJLZTZDS-IOLWVCCESA-N | ||
| Standard InChI | InChI=1S/C57H94O26/c1-21(20-73-50-42(67)40(65)37(62)32(18-58)78-50)10-15-57(72)22(2)34-31(83-57)17-30-28-9-8-26-16-27(11-13-55(26,6)29(28)12-14-56(30,34)7)77-54-49(82-52-44(69)39(64)36(61)24(4)75-52)46(71)48(33(19-59)79-54)81-53-45(70)41(66)47(25(5)76-53)80-51-43(68)38(63)35(60)23(3)74-51/h8,21-25,27-54,58-72H,9-20H2,1-7H3/t21?,22-,23-,24-,25-,27?,28?,29?,30?,31?,32+,33+,34?,35-,36-,37+,38+,39+,40-,41-,42+,43+,44+,45+,46-,47-,48+,49+,50+,51-,52-,53-,54+,55?,56?,57+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | 《Chinese Journal of Natural Medicines》 2009-05Biotransformation of Dichotomin by Pectinex BE XXL[Reference: WebLink]To study of structural modification in steroidal saponin by biotransformation technology. |

Dichotomin Dilution Calculator

Dichotomin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.8366 mL | 4.1829 mL | 8.3658 mL | 16.7316 mL | 20.9146 mL |
| 5 mM | 0.1673 mL | 0.8366 mL | 1.6732 mL | 3.3463 mL | 4.1829 mL |
| 10 mM | 0.0837 mL | 0.4183 mL | 0.8366 mL | 1.6732 mL | 2.0915 mL |
| 50 mM | 0.0167 mL | 0.0837 mL | 0.1673 mL | 0.3346 mL | 0.4183 mL |
| 100 mM | 0.0084 mL | 0.0418 mL | 0.0837 mL | 0.1673 mL | 0.2091 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 9,13-Epidioxy-8(14)-abieten-18-oic acid
Catalog No.:BCN1426
CAS No.:5309-35-3
- Morellic acid
Catalog No.:BCN3073
CAS No.:5304-71-2
- Scutebarbatine J
Catalog No.:BCN8134
CAS No.:960302-85-6
- T-5224
Catalog No.:BCC5383
CAS No.:530141-72-1
- Murralongin
Catalog No.:BCN5696
CAS No.:53011-72-6
- Salinomycin
Catalog No.:BCC1916
CAS No.:53003-10-4
- CDI (1,1′-Carbonyldiimidazole)
Catalog No.:BCC2809
CAS No.:530-62-1
- Sinapic acid
Catalog No.:BCN3539
CAS No.:530-59-6
- Syringic acid
Catalog No.:BCN5699
CAS No.:530-57-4
- 2,6-Dimethoxy-1,4-benzoquinone
Catalog No.:BCN5698
CAS No.:530-55-2
- Deoxyvasicinone
Catalog No.:BCN5697
CAS No.:530-53-0
- L-Picein
Catalog No.:BCC8336
CAS No.:530-14-3
- Androsin
Catalog No.:BCN3842
CAS No.:531-28-2
- Coniferin
Catalog No.:BCN5700
CAS No.:531-29-3
- Scopolin
Catalog No.:BCN5701
CAS No.:531-44-2
- 7-Methoxycoumarin
Catalog No.:BCN2707
CAS No.:531-59-9
- Esculin
Catalog No.:BCN5904
CAS No.:531-75-9
- Coumarin-3-Carboxylic Acid
Catalog No.:BCC9220
CAS No.:531-81-7
- 4',7-Isoflavandiol
Catalog No.:BCN2855
CAS No.:531-95-3
- Boc-Pyr-OH
Catalog No.:BCC3329
CAS No.:53100-44-0
- Rapamycin (Sirolimus)
Catalog No.:BCC3592
CAS No.:53123-88-9
- Buprenorphine hydrochloride
Catalog No.:BCC5215
CAS No.:53152-21-9
- Euscaphic acid
Catalog No.:BCN5702
CAS No.:53155-25-2
- Delphinidin-3-sambubioside chloride
Catalog No.:BCN3148
CAS No.:53158-73-9
Fall panicum ( Panicum dichotomiflorum) toxicosis in three juvenile goats.[Pubmed:30565513]
J Vet Diagn Invest. 2019 Jan;31(1):90-93.
Consumption of certain grasses belonging to the genus Panicum has been found to cause hepatogenous photosensitization and crystal-associated cholangiohepatopathy in small ruminants, and liver disease in horses, in many areas of the world. We describe herein the clinical findings, microscopic lesions, and steroidal saponin analysis of Panicum dichotomiflorum associated with fatal toxicosis in 3 juvenile goats in Nebraska. The disease presentation in our case was fulminant, with anorexia, marked icterus, and death for all affected animals in less than a week. Photosensitization was not observed. The microscopic lesions consisted of severe crystal-associated cholangiohepatopathy and nephropathy, with aggregates of clear or refractile and birefringent, acicular crystals present within bile ducts, macrophages, hepatocytes, and renal tubules. High-performance liquid chromatography-mass spectrometry of the grass samples demonstrated that Dichotomin was the major steroidal saponin present (0.89 microg/mg); protodioscin was also present (0.059 microg/mg). The findings were consistent with ingestion of steroidal saponins, and P. dichotomiflorum was identified as the predominant forage available.
P-B Desulfurization: An Enabling Method for Protein Chemical Synthesis and Site-Specific Deuteration.[Pubmed:28971554]
Angew Chem Int Ed Engl. 2017 Nov 13;56(46):14607-14611.
Cysteine-mediated native chemical ligation is a powerful method for protein chemical synthesis. Herein, we report an unprecedentedly mild system (TCEP/NaBH4 or TCEP/LiBEt3 H; TCEP=tris(2-carboxyethyl)phosphine) for chemoselective peptide desulfurization to achieve effective protein synthesis via the native chemical ligation-desulfurization approach. This method, termed P-B desulfurization, features usage of common reagents, simplicity of operation, robustness, high yields, clean conversion, and versatile functionality compatibility with complex peptides/proteins. In addition, this method can be used for incorporating deuterium into the peptides after cysteine desulfurization by running the reaction in D2 O buffer. Moreover, this method enables the clean desulfurization of peptides carrying post-translational modifications, such as phosphorylation and crotonylation. The effectiveness of this method has been demonstrated by the synthesis of the cyclic peptides Dichotomin C and E and synthetic proteins, including ubiquitin, gamma-synuclein, and histone H2A.
Cyclizing Pentapeptides: Mechanism and Application of Dehydrophenylalanine as a Traceless Turn-Inducer.[Pubmed:27973857]
Org Lett. 2017 Jan 6;19(1):114-117.
Dehydrophenylalanine is used as a traceless turn-inducer in the total synthesis of Dichotomin E. Macrocyclization of the monomer is achieved in high yields and selectivity over cyclodimerization under conditions 100 times more concentrated than previously achieved. The enamide facilitates ring closing, and Rh-catalyzed hydrogenation of the unsaturated cyclic peptide results in selective formation of the natural product or its epimer, depending on our choice of phosphine ligand. NMR analysis and molecular modeling revealed that the linear peptide adopts a left-handed alpha-turn that preorganizes the N- and C-termini toward macrocyclization.
Rapid and Sensitive Determination of the Major Steroidal Saponins of Ypsilandra thibetica Franch by Ultra High-Performance Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry.[Pubmed:27015983]
J Chromatogr Sci. 2016 Jul;54(6):1010-5.
A rapid and validated method using ultra high-performance liquid chromatography coupled with a triple quadrupole mass spectrometry (UHPLC-QQQ MS) was developed for simultaneous determination of four active steroidal saponins, i.e., Dichotomin ( 1: ), pennogenin 3-O-alpha-l-arabinofuranosyl-(1-->4)-[alpha-l-rhamnopyranosyl-(1-->2)]-beta-d-glu copyranoside ( 2: ), pennogenin 3-O-alpha-l-rhamnopyranosyl-(1-->2)-[alpha-l-rhamnopyranosyl-(1-->4)-alpha-l-rham nopyranosyl-(1-->4)]-beta-d-glucopyranoside ( 3: ) and diosgenin 3-O-alpha-l-rhamnopyranosyl-(1-->2)-[alpha-l-rhamnopyranosyl-(1-->4)-alpha-l-rham nopyranosyl-(1-->4)]-beta-d-glucopyranosidein ( 4: ), in Ypsilandra thibetica Franch. The optimized sample preparation and UHPLC-QQQ MS conditions were chosen for quantitative analysis. The separation was performed on an Agilent Zorbax Eclipse Plus C18 column (2.1 mm x 50 mm, 1.8 microm) with gradient elution of acetonitrile-0.1% formic acid in water. All calibration curves showed good linear regression (r> 0.9985) within the test range. The limits of detection and quantification were in the range of 0.02-4.40 and 0.04-22.0 ng/mL, respectively. The proposed method was applied to analyze two batches of Y. thibetica samples for target compounds within 10 min. This work promoted the quality control method for raw material or preparations of Y. thibetica.
New furostanol saponins from Allium ascalonicum L.[Pubmed:17661431]
Magn Reson Chem. 2007 Sep;45(9):725-33.
An analysis of the polar extracts from Allium ascalonicum L. led to the isolation of two new furostanol saponins (compound 1 and 2) and two known furostanol saponins (compound 3 and 4). On the basis of 1D and 2D NMR (including (1)H, (13)C NMR, (1)H--(1)H COSY, HSQC, TOCSY, HMBC, and NOESY), FAB-MS spectrometry, and chemical methods, their structures were elucidated as (25R)-26-O-beta-D-glucopyranosyl-22-hydroxy-5alpha-furost-2-one-3beta, 5, 6beta, 26-tetraol-3-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranoside (ascalonicoside C, 1), (25R)-26-O-beta-D-glucopyranosyl-22-methoxy-5alpha-furost-2-one-3beta, 5, 6beta, 26-tetraol- 3-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranoside (ascalonicoside D, 2), (25R)-26-O-beta-D-glucopyranosyl-22-hydroxy-5-ene-furostan-3beta, 26-diol-3-O-alpha-L-rhamnopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->4)-[alph a-L-rhamnopyranosyl-(1-->2)]-beta-D-glucopyranoside (Dichotomin, 3), and (25R)-26-O-beta-D-glucopyranosyl-22-hydroxy-5-ene-furostan-3beta, 26-diol-3-O-alpha-L-rhamnopyranosyl-(1-->2)-[alpha-L-arabinofuranosyl-(1-->4)]-be ta-D- glucopyranoside (parisaponin I, 4).
Dichotomins J and K, vasodilator cyclic peptides from Stellaria dichotoma.[Pubmed:16309326]
J Nat Prod. 2005 Nov;68(11):1686-8.
Two new cyclic peptides, Dichotomins J (1) and K (2), have been isolated from the roots of Stellaria dichotoma, and their structures were elucidated by chemical degradation and extensive 2D NMR methods. Dichotomins J (1) and K (2) showed a moderate vasorelaxant effect on rat aorta.
Cyclic octapeptides from Stellaria dichotoma var. lanceolata.[Pubmed:9195763]
Phytochemistry. 1997 Jun;45(4):841-5.
Two new cyclic octapeptides, Dichotomin H, cyclo(-Ala-Pro-Thr-Phe-Tyr-P ro-Leu-Ile-), and Dichotomin I, cyclo(-Val-Pro-Thr-Phe-Tyr-Pro-Leu-Ile-) have been isolated from the roots of Stellaria dichotoma L. var lanceolata Bge., and their structures were elucidated by extensive two-dimensional NMR methods and chemical degradation.


