EtofenamateNon-steroidal anti-inflammatory drug CAS# 30544-47-9 |
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 30544-47-9 | SDF | Download SDF |
| PubChem ID | 35375 | Appearance | Powder |
| Formula | C18H18F3NO4 | M.Wt | 369.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (135.38 mM) *"≥" means soluble, but saturation unknown. | ||
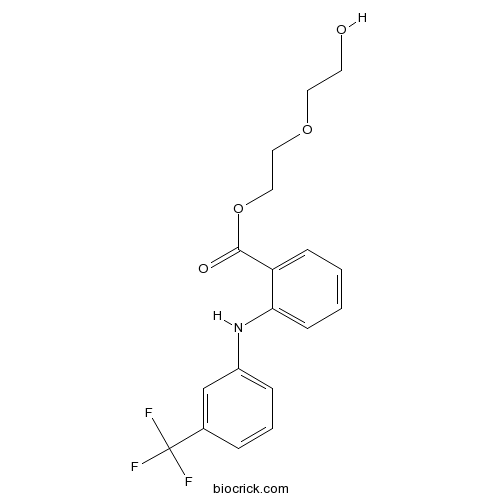
| Chemical Name | 2-(2-hydroxyethoxy)ethyl 2-[3-(trifluoromethyl)anilino]benzoate | ||
| SMILES | C1=CC=C(C(=C1)C(=O)OCCOCCO)NC2=CC=CC(=C2)C(F)(F)F | ||
| Standard InChIKey | XILVEPYQJIOVNB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H18F3NO4/c19-18(20,21)13-4-3-5-14(12-13)22-16-7-2-1-6-15(16)17(24)26-11-10-25-9-8-23/h1-7,12,22-23H,8-11H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Etofenamate is a non-steroidal anti-inflammatory drug used for the treatment joint and muscular pain. References: | |||||

Etofenamate Dilution Calculator

Etofenamate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7075 mL | 13.5377 mL | 27.0753 mL | 54.1506 mL | 67.6883 mL |
| 5 mM | 0.5415 mL | 2.7075 mL | 5.4151 mL | 10.8301 mL | 13.5377 mL |
| 10 mM | 0.2708 mL | 1.3538 mL | 2.7075 mL | 5.4151 mL | 6.7688 mL |
| 50 mM | 0.0542 mL | 0.2708 mL | 0.5415 mL | 1.083 mL | 1.3538 mL |
| 100 mM | 0.0271 mL | 0.1354 mL | 0.2708 mL | 0.5415 mL | 0.6769 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Etofenamate is a non-steroidal anti-inflammatory drug used for the treatment joint and muscular pain.
- H-Cys(Bzl)-OH
Catalog No.:BCC2906
CAS No.:3054-01-1
- 5'-Demethylaquillochin
Catalog No.:BCN5221
CAS No.:305364-91-4
- Caulilexin C
Catalog No.:BCN3960
CAS No.:30536-48-2
- SL-327
Catalog No.:BCC1123
CAS No.:305350-87-2
- Zidovudine
Catalog No.:BCC5024
CAS No.:30516-87-1
- Licoricidin
Catalog No.:BCN6679
CAS No.:30508-27-1
- L-carnosine
Catalog No.:BCN3803
CAS No.:305-84-0
- Chlorambucil
Catalog No.:BCC5351
CAS No.:305-03-3
- 6,7-Dihydroxycoumarin
Catalog No.:BCN5905
CAS No.:305-01-1
- SANT-1
Catalog No.:BCC3941
CAS No.:304909-07-7
- AGK 2
Catalog No.:BCC7609
CAS No.:304896-28-4
- Alisol C
Catalog No.:BCN3458
CAS No.:30489-27-1
- Bisabolangelone
Catalog No.:BCN8094
CAS No.:30557-81-4
- Stavudine (d4T)
Catalog No.:BCC5028
CAS No.:3056-17-5
- Acephate
Catalog No.:BCC7555
CAS No.:30560-19-1
- Geldanamycin
Catalog No.:BCC2125
CAS No.:30562-34-6
- H-Asp(OtBu)-OH
Catalog No.:BCC2891
CAS No.:3057-74-7
- 3,9-Bis(2-cyanoethyl)-2,4,8,10-tetraoxaspiro[5.5]undecane
Catalog No.:BCC8599
CAS No.:3058-04-6
- SC 79
Catalog No.:BCC6246
CAS No.:305834-79-1
- Homovanillic acid
Catalog No.:BCN1253
CAS No.:306-08-1
- Spermine tetrahydrochloride
Catalog No.:BCC6864
CAS No.:306-67-2
- 2,2-Diphenylglycine
Catalog No.:BCC8496
CAS No.:3060-50-2
- AVE 0991 sodium salt
Catalog No.:BCC4222
CAS No.:306288-04-0
- (20S)-Protopanaxdiol
Catalog No.:BCN1254
CAS No.:30636-90-9
Novel Drug Delivery System for Dermal Uptake of Etofenamate: Semisolid SLN Dispersion.[Pubmed:27501715]
Curr Drug Deliv. 2017;14(3):386-393.
BACKGROUND: Semisolid SLNs are novel strategy for dermal drug administration instead of incorporating the SLN dispersions into conventional semisolids. Etofenamate loaded semisolid SLNs were successfully prepared and in vitro characterization of formulations were performed in our previous study. The present study is an attempt to evaluate the dermal behavior of the semisolid SLNs selected on the basis of previous research and investigate the properties in terms of the convenience for topical applications. OBJECTIVE: The objective of this study is to evaluate the skin penetration characteristics of semisolid SLN formulations. The occlusive and mechanical properties of semisolid SLNs were also evaluated because of their impression on the dermal behavior of the formulations. METHOD: The occlusive properties were investigated by in vitro occlusion test. Texture analysis was performed to define the hardness, compressibility, adhesiveness, cohesiveness and elasticity of the formulations. Rat skin was chosen to evaluate the ex vivo penetration of Etofenamate loaded semisolid SLNs and commercial gel product. Coumarin-6 was used to visualize the dermal distribution of the semisolid SLN formulations. For monitorizing the penetration of coumarin-6 into the skin samples Confocal Laser Scanning Microscopy was employed. RESULTS: The occlusive and mechanical properties of C1 coded semisolid SLN formulation were found more favorable in comparison with P1. The cumulative Etofenamate amount in skin samples was found to be 39.88 +/- 1.50 mug/cm2 for C1 and 30.56 +/- 2.10 mug/cm2 for P1 coded formulations. According to CLSM images, greater fluorescence intensities and deeper skin penetrations were obtained with both of the semisolid SLNs in comparison to plain Carbopol gel. CONCLUSION: It can be concluded that the semisolid SLNs are promising alternative dermal drug delivery systems to the conventional dosage forms.
Analytical Quality by Design Approach in RP-HPLC Method Development for the Assay of Etofenamate in Dosage Forms.[Pubmed:26997704]
Indian J Pharm Sci. 2015 Nov-Dec;77(6):751-7.
By considering the current regulatory requirement for an analytical method development, a reversed phase high performance liquid chromatographic method for routine analysis of Etofenamate in dosage form has been optimized using analytical quality by design approach. Unlike routine approach, the present study was initiated with understanding of quality target product profile, analytical target profile and risk assessment for method variables that affect the method response. A liquid chromatography system equipped with a C18 column (250x4.6 mm, 5 mu), a binary pump and photodiode array detector were used in this work. The experiments were conducted based on plan by central composite design, which could save time, reagents and other resources. Sigma Tech software was used to plan and analyses the experimental observations and obtain quadratic process model. The process model was used for predictive solution for retention time. The predicted data from contour diagram for retention time were verified actually and it satisfied with actual experimental data. The optimized method was achieved at 1.2 ml/min flow rate of using mobile phase composition of methanol and 0.2% triethylamine in water at 85:15, % v/v, pH adjusted to 6.5. The method was validated and verified for targeted method performances, robustness and system suitability during method transfer.
Effectiveness of etofenamate for treatment of knee osteoarthritis: a randomized controlled trial.[Pubmed:27881922]
Ther Clin Risk Manag. 2016 Nov 14;12:1693-1699.
The intramuscular application of Etofenamate in the treatment of knee osteoarthritis was not observed in the existing English language literature. The objectives of this study were to compare the efficacy of Etofenamate versus hyaluronic acid (HA) in reducing joint pain and functional improvement for mild to moderate knee osteoarthritis. The patients were randomly divided into Etofenamate (n=29) and HA (n=30) groups. Intramuscular Etofenamate injection was administered as a series of seven intramuscular injections at intervals of 1 day. Intra-articular HA injection was administered as a series of three intra-articular injections at intervals of 1 week. Clinical evaluation was made before the first injection and again both 6 and 12 months after the last injection. The evaluation consisted of patient-assessed pain on a visual analog scale (VAS) and on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Statistical significance was found for the Etofenamate group when comparing preinjection with 12 months postinjection VAS scores (P<0.05). Statistical significance was also found for the HA group when comparing preinjection with 12 months postinjection VAS and WOMAC scores (P<0.05). However, there was no significant difference between the Etofenamate and HA groups in terms of VAS or WOMAC scores measured at 12 months after injection (P>0.05). Results from this study indicated that, Etofenamate treatment was not significantly more effective than HA treatment. However, both methods were effective and successful in treating knee osteoarthritis.


