Caulilexin CCAS# 30536-48-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 30536-48-2 | SDF | Download SDF |
| PubChem ID | 11954881 | Appearance | Oil |
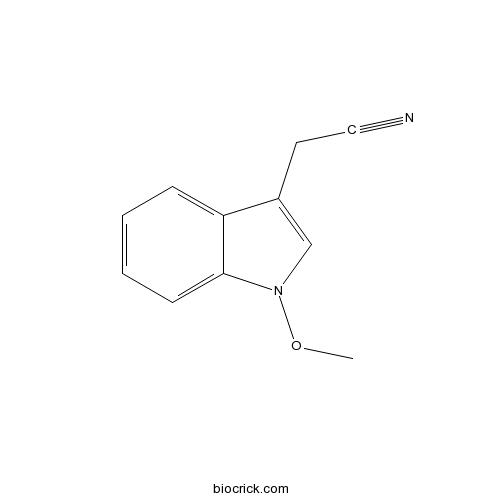
| Formula | C11H10N2O | M.Wt | 186.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(1-methoxyindol-3-yl)acetonitrile | ||
| SMILES | CON1C=C(C2=CC=CC=C21)CC#N | ||
| Standard InChIKey | LIJIPBYXIXTNLE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H10N2O/c1-14-13-8-9(6-7-12)10-4-2-3-5-11(10)13/h2-5,8H,6H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Caulilexin C shows inhibitory activity on human Acyl CoA: cholesterol transferase I (hACATI) and on human Acyl CoA: cholesterol transferase 2 (hACAT2) at 100 mug/ml. |

Caulilexin C Dilution Calculator

Caulilexin C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.3706 mL | 26.8528 mL | 53.7057 mL | 107.4114 mL | 134.2642 mL |
| 5 mM | 1.0741 mL | 5.3706 mL | 10.7411 mL | 21.4823 mL | 26.8528 mL |
| 10 mM | 0.5371 mL | 2.6853 mL | 5.3706 mL | 10.7411 mL | 13.4264 mL |
| 50 mM | 0.1074 mL | 0.5371 mL | 1.0741 mL | 2.1482 mL | 2.6853 mL |
| 100 mM | 0.0537 mL | 0.2685 mL | 0.5371 mL | 1.0741 mL | 1.3426 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SL-327
Catalog No.:BCC1123
CAS No.:305350-87-2
- Zidovudine
Catalog No.:BCC5024
CAS No.:30516-87-1
- Licoricidin
Catalog No.:BCN6679
CAS No.:30508-27-1
- L-carnosine
Catalog No.:BCN3803
CAS No.:305-84-0
- Chlorambucil
Catalog No.:BCC5351
CAS No.:305-03-3
- 6,7-Dihydroxycoumarin
Catalog No.:BCN5905
CAS No.:305-01-1
- SANT-1
Catalog No.:BCC3941
CAS No.:304909-07-7
- AGK 2
Catalog No.:BCC7609
CAS No.:304896-28-4
- Alisol C
Catalog No.:BCN3458
CAS No.:30489-27-1
- Tanaproget
Catalog No.:BCC1984
CAS No.:304853-42-7
- Mearnsitrin
Catalog No.:BCN5220
CAS No.:30484-88-9
- Flunarizine 2HCl
Catalog No.:BCC4398
CAS No.:30484-77-6
- 5'-Demethylaquillochin
Catalog No.:BCN5221
CAS No.:305364-91-4
- H-Cys(Bzl)-OH
Catalog No.:BCC2906
CAS No.:3054-01-1
- Etofenamate
Catalog No.:BCC1563
CAS No.:30544-47-9
- Bisabolangelone
Catalog No.:BCN8094
CAS No.:30557-81-4
- Stavudine (d4T)
Catalog No.:BCC5028
CAS No.:3056-17-5
- Acephate
Catalog No.:BCC7555
CAS No.:30560-19-1
- Geldanamycin
Catalog No.:BCC2125
CAS No.:30562-34-6
- H-Asp(OtBu)-OH
Catalog No.:BCC2891
CAS No.:3057-74-7
- 3,9-Bis(2-cyanoethyl)-2,4,8,10-tetraoxaspiro[5.5]undecane
Catalog No.:BCC8599
CAS No.:3058-04-6
- SC 79
Catalog No.:BCC6246
CAS No.:305834-79-1
- Homovanillic acid
Catalog No.:BCN1253
CAS No.:306-08-1
- Spermine tetrahydrochloride
Catalog No.:BCC6864
CAS No.:306-67-2
Interaction of cruciferous phytoanticipins with plant fungal pathogens: indole glucosinolates are not metabolized but the corresponding desulfo-derivatives and nitriles are.[Pubmed:21920565]
Phytochemistry. 2011 Dec;72(18):2308-16.
Glucosinolates represent a large group of plant natural products long known for diverse and fascinating physiological functions and activities. Despite the relevance and huge interest on the roles of indole glucosinolates in plant defense, little is known about their direct interaction with microbial plant pathogens. Toward this end, the metabolism of indolyl glucosinolates, their corresponding desulfo-derivatives, and derived metabolites, by three fungal species pathogenic on crucifers was investigated. While glucobrassicin, 1-methoxyglucobrassicin, 4-methoxyglucobrassicin were not metabolized by the pathogenic fungi Alternaria brassicicola, Rhizoctonia solani and Sclerotinia sclerotiorum, the corresponding desulfo-derivatives were metabolized to indolyl-3-acetonitrile, Caulilexin C (1-methoxyindolyl-3-acetonitrile) and arvelexin (4-methoxyindolyl-3-acetonitrile) by R. solani and S. sclerotiorum, but not by A. brassicicola. That is, desulfo-glucosinolates were metabolized by two non-host-selective pathogens, but not by a host-selective. Indolyl-3-acetonitrile, Caulilexin C and arvelexin were metabolized to the corresponding indole-3-carboxylic acids. Indolyl-3-acetonitriles displayed higher inhibitory activity than indole desulfo-glucosinolates. Indolyl-3-methanol displayed antifungal activity and was metabolized by A. brassicicola and R. solani to the less antifungal compounds indole-3-carboxaldehyde and indole-3-carboxylic acid. Diindolyl-3-methane was strongly antifungal and stable in fungal cultures, but ascorbigen was not stable in solution and displayed low antifungal activity; neither compound appeared to be metabolized by any of the three fungal species. The cell-free extracts of mycelia of A. brassicicola displayed low myrosinase activity using glucobrassicin as substrate, but myrosinase activity was not detectable in mycelia of either R. solani or S. sclerotiorum.


