6,7-DihydroxycoumarinCAS# 305-01-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 305-01-1 | SDF | Download SDF |
| PubChem ID | 5281416 | Appearance | White-pale yellow powder |
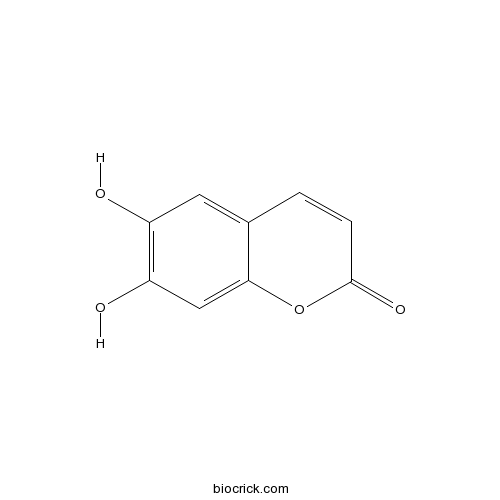
| Formula | C9H6O4 | M.Wt | 178.14 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Synonyms | Aesculetin; Cichorigenin; Cichoriin aglycone; 6,7-Dihydroxycoumarin; Esculetol; Esculin aglycone | ||
| Solubility | DMSO : 250 mg/mL (1403.39 mM; Need ultrasonic) | ||
| Chemical Name | 6,7-dihydroxychromen-2-one | ||
| SMILES | C1=CC(=O)OC2=CC(=C(C=C21)O)O | ||
| Standard InChIKey | ILEDWLMCKZNDJK-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 6,7-Dihydroxycoumarin(Esculetin) has various biological and pharmaceutical properties including anti-edema, anti-inflammatory, anti-tumour, hepatoprotective, anti-osteoarthritis and anti-rheumatoid arthritis effects. It inhibits lipoxygenases (LOs), p42/44 MAPK activation, PI3-kinase activation, as well as NF-kappaB and AP-1 activation, it exhibits competitive inhibition against the oxidation of 3-(3,4-dihydroxyphenyl)- alanine by mushroom, the IC50 value of is 43 microM. |
| Targets | HO-1 | NO | AP-1 | MCP-1 | TNF-α | PPAR | NF-kB | PI3K |
| In vitro | Esculetin (6,7-dihydroxycoumarin): a potential cancer chemopreventive agent through suppression of Sp1 in oral squamous cancer cells.[Pubmed: 25310400]Int J Oncol. 2015 Jan;46(1):265-71.Esculetin (6,7-Dihydroxycoumarin), a coumarin compound, is known to inhibit proliferation and induce apoptosis in several types of human cancer cells and is regarded as a promising chemotherapeutic agent.
Esculetin inhibits the inflammatory response by inducing heme oxygenase-1 in cocultured macrophages and adipocytes.[Pubmed: 25088305]Food Funct. 2014 Sep;5(9):2371-7.
Esculetin suppresses proteoglycan metabolism by inhibiting the production of matrix metalloproteinases in rabbit chondrocytes.[Pubmed: 10334506]Eur. J. Pharmacol., 1999, 370(3):297-305The possible mechanism of the chondroprotective effect of 6,7-Dihydroxycoumarin (esculetin) was investigated using primary cultures of rabbit articular chondrocytes.
|
| Kinase Assay | Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L.[Pubmed: 12723615 ]Esculetin inhibits Ras-mediated cell proliferation and attenuates vascular restenosis following angioplasty in rats.[Pubmed: 12781342]Biochem Pharmacol. 2003 Jun 1;65(11):1897-905.The proliferation of vascular smooth muscle cells (VSMCs) induced by injury to the intima of arteries is an important etiologic factor in vascular proliferative disorders such as atherosclerosis and restenosis. Esculetin, derived from the Chinese herb Artemisia scoparia, is well known as a lipoxygenase inhibitor.
Biosci Biotechnol Biochem. 2003 Mar;67(3):631-4.A tyrosinase inhibitor was isolated from the seeds of Euphorbia lathyris L. by bioassay-guided fractionation and purification, using silica gel column chromatography. It was identified as esculetin (6,7-Dihydroxycoumarin)by comparing its physical properties and spectral data with those of an authentic sample. The IC50 value of esculetin(6,7-Dihydroxycoumarin) in the mushroom tyrosinase activity test was 43 microM. The kinetic study indicates that esculetin(6,7-Dihydroxycoumarin) exhibited competitive inhibition against the oxidation of 3-(3,4-dihydroxyphenyl)-alanine by mushroom tyrosinase. The structure-activity relationships among five esculetin(6,7-Dihydroxycoumarin) analogs suggest that hydroxyl groups at the C6 and C7 positions of the coumarin skeleton played an important role in the expression of tyrosinase inhibitory activity. |
| Cell Research | Esculetin induces apoptosis and inhibits adipogenesis in 3T3-L1 cells.[Pubmed: 17062797 ]Obesity (Silver Spring). 2006 Oct;14(10):1691-9.Cell lines:3T3-L1 mouse embryo fibroblasts |
| Animal Research | Esculetin(6,7-Dihydroxycoumarin), a coumarin derivative, exerts in vitro and in vivo antiproliferative activity against hepatocellular carcinoma by initiating a mitochondrial-dependent apoptosis pathway.[Pubmed: 25517918 ]Braz J Med Biol Res. 2015 Mar;48(3):245-53.Animal Models: C57BL/6J mice implanted with Hepa1-6 cells |

6,7-Dihydroxycoumarin Dilution Calculator

6,7-Dihydroxycoumarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6136 mL | 28.0678 mL | 56.1356 mL | 112.2712 mL | 140.3391 mL |
| 5 mM | 1.1227 mL | 5.6136 mL | 11.2271 mL | 22.4542 mL | 28.0678 mL |
| 10 mM | 0.5614 mL | 2.8068 mL | 5.6136 mL | 11.2271 mL | 14.0339 mL |
| 50 mM | 0.1123 mL | 0.5614 mL | 1.1227 mL | 2.2454 mL | 2.8068 mL |
| 100 mM | 0.0561 mL | 0.2807 mL | 0.5614 mL | 1.1227 mL | 1.4034 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SANT-1
Catalog No.:BCC3941
CAS No.:304909-07-7
- AGK 2
Catalog No.:BCC7609
CAS No.:304896-28-4
- Alisol C
Catalog No.:BCN3458
CAS No.:30489-27-1
- Tanaproget
Catalog No.:BCC1984
CAS No.:304853-42-7
- Mearnsitrin
Catalog No.:BCN5220
CAS No.:30484-88-9
- Flunarizine 2HCl
Catalog No.:BCC4398
CAS No.:30484-77-6
- S 24795
Catalog No.:BCC7700
CAS No.:304679-75-2
- Xerophilusin G
Catalog No.:BCN5219
CAS No.:304642-94-2
- Theaflavin 3,3'-di-O-gallate
Catalog No.:BCN5920
CAS No.:30462-35-2
- Theaflavin-3-gallate
Catalog No.:BCN2316
CAS No.:30462-34-1
- Cyclomusalenone
Catalog No.:BCN4654
CAS No.:30452-60-9
- AVE 0991
Catalog No.:BCC4032
CAS No.:304462-19-9
- Chlorambucil
Catalog No.:BCC5351
CAS No.:305-03-3
- L-carnosine
Catalog No.:BCN3803
CAS No.:305-84-0
- Licoricidin
Catalog No.:BCN6679
CAS No.:30508-27-1
- Zidovudine
Catalog No.:BCC5024
CAS No.:30516-87-1
- SL-327
Catalog No.:BCC1123
CAS No.:305350-87-2
- Caulilexin C
Catalog No.:BCN3960
CAS No.:30536-48-2
- 5'-Demethylaquillochin
Catalog No.:BCN5221
CAS No.:305364-91-4
- H-Cys(Bzl)-OH
Catalog No.:BCC2906
CAS No.:3054-01-1
- Etofenamate
Catalog No.:BCC1563
CAS No.:30544-47-9
- Bisabolangelone
Catalog No.:BCN8094
CAS No.:30557-81-4
- Stavudine (d4T)
Catalog No.:BCC5028
CAS No.:3056-17-5
- Acephate
Catalog No.:BCC7555
CAS No.:30560-19-1
Esculetin suppresses proteoglycan metabolism by inhibiting the production of matrix metalloproteinases in rabbit chondrocytes.[Pubmed:10334506]
Eur J Pharmacol. 1999 Apr 16;370(3):297-305.
The possible mechanism of the chondroprotective effect of 6,7-Dihydroxycoumarin (esculetin) was investigated using primary cultures of rabbit articular chondrocytes. Esculetin (EST) significantly suppressed the proteoglycan depletion and the release of pulse-labeled [35S]proteoglycan from the matrix layer of rabbit chondrocytes treated with recombinant human interleukin-1alpha. The matrix metalloproteinase inhibitor, 1,10-phenanthroline, also blocked the proteoglycan depletion and [35S]proteoglycan release. From these results, it is likely that recombinant human interleukin-1alpha-induced proteoglycan depletion is mediated by matrix metalloproteinases. Although esculetin did not directly inhibit collagenolytic activity in the culture media, it significantly suppressed the production of pro-matrix metalloproteinase-1/interstitial procollagenase and pro-matrix metalloproteinase-3/prostromelysin 1, accompanied by a decrease in the steady-state levels of their mRNAs. These results suggest that esculetin is a therapeutically effective candidate for inhibition of cartilage destruction in osteoarthritis and rheumatoid arthritis.
Esculetin (6,7-dihydroxycoumarin): a potential cancer chemopreventive agent through suppression of Sp1 in oral squamous cancer cells.[Pubmed:25310400]
Int J Oncol. 2015 Jan;46(1):265-71.
Esculetin (6,7-Dihydroxycoumarin), a coumarin compound, is known to inhibit proliferation and induce apoptosis in several types of human cancer cells and is regarded as a promising chemotherapeutic agent. The purpose of the present study was to investigate the anti-proliferative effects of esculetin on two oral squamous cell carcinoma (OSCC) cell lines, HN22 and HSC4, through regulation of specificity protein 1 (Sp1). We examined the apoptotic effects of esculetin were measured by MTS assay, DAPI staining, Annexin V, PI staining, RT-PCR, western blot analysis and immunocytochemistry in HN22 and HSC4 cells. Taken together, the results of the present study indicate that esculetin had anti-proliferative effect on the growth of OSCC cells (HN22 and HSC4) in a dose- and time-dependent manner. The treatment of HN22 and HSC4 cells with esculetin led to a significant reduction in growth and induced apoptosis, followed by the regulation of Sp1 and Sp1 regulatory protein. This indicates that esculetin inhibited cell growth and induced apoptosis by suppressing Sp1 in HN22 and HSC4 cells, suggesting it to be a potent anticancer drug candidate for oral cancer.
Esculetin inhibits the inflammatory response by inducing heme oxygenase-1 in cocultured macrophages and adipocytes.[Pubmed:25088305]
Food Funct. 2014 Sep;5(9):2371-7.
Obesity is associated with chronic low-grade inflammation of adipose tissue. In this study, we investigated the anti-inflammatory effects of esculetin (ECT) through up-regulation of heme oxygenase-1 (HO-1) in cocultured macrophages and adipocytes. RAW264.7 macrophages and differentiated 3T3-L1 adipocytes were cocultured in serum-free Dulbecco's modified Eagle's medium with or without ECT for 24 h. Nitric oxide (NO), tumor necrosis factor-alpha (TNF-alpha), and monocyte chemoattractant protein-1 (MCP-1) production was measured in the coculture supernatant. ECT decreased the secretion of NO, TNF-alpha, and MCP-1. The expression of adipogenic proteins, including peroxisome proliferator-activated receptors gamma (PPARgamma) and CCAAT/enhancer binding protein alpha (C/EBPalpha) in cocultured adipocytes and inducible nitric oxide synthase (iNOS) in cocultured macrophages, was inhibited by ECT. Additionally, HO-1 expression was induced in cocultured macrophages and adipocytes. Silencing of HO-1 expression increased the production of NO, TNF-alpha, and MCP-1 in cocultured cells, in spite of the presence of ECT. This study demonstrated that ECT exhibited anti-inflammatory properties by inhibiting the production of proinflammatory cytokines in the interaction between adipocytes and macrophages through HO-1 expression. ECT may have the potential to improve chronic inflammation in obesity.
Esculetin inhibits Ras-mediated cell proliferation and attenuates vascular restenosis following angioplasty in rats.[Pubmed:12781342]
Biochem Pharmacol. 2003 Jun 1;65(11):1897-905.
The proliferation of vascular smooth muscle cells (VSMCs) induced by injury to the intima of arteries is an important etiologic factor in vascular proliferative disorders such as atherosclerosis and restenosis. Esculetin, derived from the Chinese herb Artemisia scoparia, is well known as a lipoxygenase inhibitor. We have investigated the inhibitory effects of esculetin on VSMC proliferation and intimal hyperplasia by balloon angioplasty in the rat. We determined, using [3H]thymidine incorporation and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, that esculetin inhibited the proliferation of VSMCs via a lipoxygenase-independent pathway. Three predominant signaling pathways were identified to be inhibited by esculetin: (a) the activation of p42/44 mitogen-activated protein kinase (MAPK) and the downstream effectors of c-fos and c-jun immediate early genes by means of western and reverse transcription-polymerase chain reaction (RT-PCR) analyses; (b) the activation of nuclear factor-kappaB (NF-kappaB) and activator protein-1 (AP-1), using the electrophoretic mobility shift assay; and (c) the activation of phosphoinositide 3-kinase (PI 3-kinase) and cell cycle progression, by western blot analysis and flow cytometric detection. Furthermore, esculetin also profoundly inhibited Ras activation, a shared upstream event of the above signaling cascades. In vascular injury studies, intraperitoneal administration of esculetin significantly suppressed intimal hyperplasia induced by balloon angioplasty. We conclude that esculetin blocks cell proliferation via the inhibition of an upstream effector of Ras and downstream events including p42/44 MAPK activation, PI 3-kinase activation, immediate early gene expression, as well as NF-kappaB and AP-1 activation. It also inhibits intimal hyperplasia after balloon vascular injury in the rat, indicating the therapeutic potential for treating restenosis after arterial injury.
Esculetin induces apoptosis and inhibits adipogenesis in 3T3-L1 cells.[Pubmed:17062797]
Obesity (Silver Spring). 2006 Oct;14(10):1691-9.
OBJECTIVE: To determine the effects of esculetin, a plant phenolic compound with apoptotic activity in cancer cells, on 3T3-L1 adipocyte apoptosis and adipogenesis. RESEARCH METHODS AND PROCEDURES: 3T3-L1 pre-confluent preadipocytes and lipid-filled adipocytes were incubated with esculetin (0 to 800 microM) for up to 48 hours. Viability was determined using the Cell Titer 96 Aqueous One Solution cell proliferation assay; apoptosis was quantified by measurement of single-stranded DNA. Post-confluent preadipocytes were incubated with esculetin for up to 6 days during maturation. Adipogenesis was quantified by measuring lipid content using Nile Red dye; cells were also stained with Oil Red O for visual confirmation of effects on lipid accumulation. RESULTS: In mature adipocytes, esculetin caused a time- and dose-related increase in adipocyte apoptosis and a decrease in viability. Apoptosis was increased after only 6 hours by 400 and 800 microM esculetin (p < 0.05), and after 48 hours, as little as 50 microM esculetin increased apoptosis (p < 0.05). In preadipocytes, apoptosis was detectable only after 48 hours (p < 0.05) with 200 microM esculetin and higher concentrations. However, results of the cell viability assay indicated a reduction in preadipocyte number in a time- and dose-related manner, beginning as early as 6 hours with 400 and 800 microM esculetin (p < 0.05). Esculetin also inhibited adipogenesis of 3T3-L1 preadipocytes. Esculetin-mediated inhibition of adipocyte differentiation occurred during the early, intermediate, and late stages of the differentiation process. In addition, esculetin induced apoptosis during the late stage of differentiation. DISCUSSION: These findings suggest that esculetin can alter fat cell number by direct effects on cell viability, adipogenesis, and apoptosis in 3T3-L1 cells.
Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver.[Pubmed:11097384]
Arch Toxicol. 2000 Oct;74(8):467-72.
Increasing evidence regarding free radical-generating agents and inflammatory processes suggests that accumulation of reactive oxygen species can cause hepatotoxicity. A short-chain analog of lipid hydroperoxide, t-butyl hydroperoxide (t-BHP), can be metabolized to free radical intermediates by cytochrome P-450 in hepatocytes, which in turn can initiate lipid peroxidation, affect cell integrity and result in cell injury. In this study, we used t-BHP to induce hepatotoxicity in vitro and in vivo and determined the antioxidative bioactivity of esculetin, a coumarin compound. Our investigations showed that pretreatment with esculetin (5-20 microg/ml) significantly decreased the leakage of lactate dehydrogenase (LDH) and alanine transaminase (ALT), and also decreased the formation of malondialdehyde (MDA) in primary cultured rat hepatocytes induced by a 30-min treatment with t-BHP. An in vivo study in rats showed that pretreatment with esculetin (i.p.) at concentrations of 0.5 and 5 mg/kg for 5 days before a single i.p. dose of t-BHP (0.1 mmol/kg) significantly lowered the serum levels of the hepatic enzyme markers (ALT and AST) and reduced oxidative stress in the liver. Histopathological evaluation of the rat livers revealed that esculetin reduced the incidence of liver lesions induced by t-BHP, including hepatocyte swelling, leukocyte infiltration, and necrosis. Based on the results described above, we speculate that esculetin may play a chemopreventive role via reducing oxidative stress in living systems.
Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L.[Pubmed:12723615]
Biosci Biotechnol Biochem. 2003 Mar;67(3):631-4.
A tyrosinase inhibitor was isolated from the seeds of Euphorbia lathyris L. by bioassay-guided fractionation and purification, using silica gel column chromatography. It was identified as esculetin by comparing its physical properties and spectral data with those of an authentic sample. The IC50 value of esculetin in the mushroom tyrosinase activity test was 43 microM. The kinetic study indicates that esculetin exhibited competitive inhibition against the oxidation of 3-(3,4-dihydroxyphenyl)-alanine by mushroom tyrosinase. The structure-activity relationships among five esculetin analogs suggest that hydroxyl groups at the C6 and C7 positions of the coumarin skeleton played an important role in the expression of tyrosinase inhibitory activity.


