Betulinic acid methyl esterCAS# 2259-06-5 |
Quality Control & MSDS
Number of papers citing our products

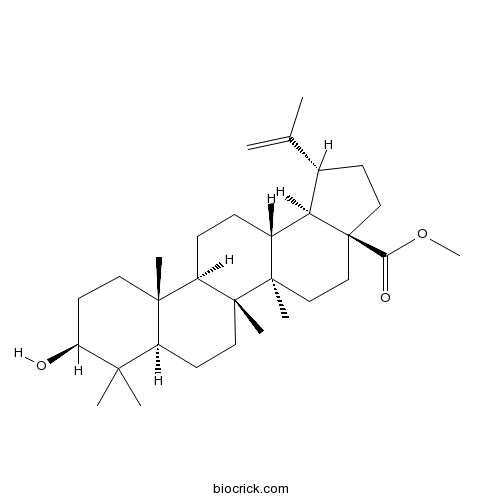
Chemical structure

3D structure
| Cas No. | 2259-06-5 | SDF | Download SDF |
| PubChem ID | 73493 | Appearance | Powder |
| Formula | C31H50O3 | M.Wt | 470.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (1~{R},3~{a}~{S},5~{a}~{R},5~{b}~{R},7~{a}~{R},9~{S},11~{a}~{R},11~{b}~{R},13~{a}~{R},13~{b}~{R})-9-hydroxy-5~{a},5~{b},8,8,11~{a}-pentamethyl-1-prop-1-en-2-yl-1,2,3,4,5,6,7,7~{a},9,10,11,11~{b},12,13,13~{a},13~{b}-hexadecahydrocyclopenta[a]chrysene-3~{a}-carboxylate | ||
| SMILES | CC(=C)C1CCC2(C1C3CCC4C5(CCC(C(C5CCC4(C3(CC2)C)C)(C)C)O)C)C(=O)OC | ||
| Standard InChIKey | XNZIMRUZBOZIBC-JVRMVBBZSA-N | ||
| Standard InChI | InChI=1S/C31H50O3/c1-19(2)20-11-16-31(26(33)34-8)18-17-29(6)21(25(20)31)9-10-23-28(5)14-13-24(32)27(3,4)22(28)12-15-30(23,29)7/h20-25,32H,1,9-18H2,2-8H3/t20-,21+,22-,23+,24-,25+,28-,29+,30+,31-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Betulinic acid methyl ester showed antiplasmodial activity against chloroquine-resistant Plasmodium falciparum parasites in vitro.It also inhibited B16 2F2 cell proliferation by induction of apoptosis. |
| Targets | Antifection |
| In vitro | Antimalarial activity of betulinic acid and derivatives in vitro against Plasmodium falciparum and in vivo in P. berghei-infected mice.[Pubmed: 19367418]Parasitol Res. 2009 Jul;105(1):275-9.Malaria is one of the most important tropical diseases and mainly affects populations living in developing countries. Reduced sensitivity of Plasmodium sp. to formerly recommended antimalarial drugs places an increasing burden on malaria control programs as well as on national health systems in endemic countries. The present study aims to evaluate the antimalarial activity of betulinic acid and its derivative compounds, betulonic acid, betulinic acid acetate, Betulinic acid methyl ester, and Betulinic acid methyl ester acetate. Differentiation- and apoptosis-inducing activities by pentacyclic triterpenes on a mouse melanoma cell line.[Pubmed: 12027734 ]J Nat Prod. 2002 May;65(5):645-8.In a study to investigate the relationship between the chemical structure and the differentiation-inducing activity of pentacyclic triterpenes, several lupane, oleanane, and ursane triterpenes were prepared and their effects on B16 2F2 melanoma cell differentiation and growth were examined.
|

Betulinic acid methyl ester Dilution Calculator

Betulinic acid methyl ester Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1245 mL | 10.6225 mL | 21.245 mL | 42.4899 mL | 53.1124 mL |
| 5 mM | 0.4249 mL | 2.1245 mL | 4.249 mL | 8.498 mL | 10.6225 mL |
| 10 mM | 0.2124 mL | 1.0622 mL | 2.1245 mL | 4.249 mL | 5.3112 mL |
| 50 mM | 0.0425 mL | 0.2124 mL | 0.4249 mL | 0.8498 mL | 1.0622 mL |
| 100 mM | 0.0212 mL | 0.1062 mL | 0.2124 mL | 0.4249 mL | 0.5311 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lucidenic acid L
Catalog No.:BCN6389
CAS No.:110267-45-3
- Neochebulagic acid
Catalog No.:BCN7368
CAS No.:28196-46-5
- Chelidimerine
Catalog No.:BCN8410
CAS No.:39110-99-1
- Tuberosin
Catalog No.:BCN8735
CAS No.:41347-45-9
- Picropodopyllotoxone
Catalog No.:BCN7574
CAS No.:477-48-5
- 6''-O-Acetylsaikosaponin D
Catalog No.:BCN6392
CAS No.:64340-45-0
- Polygalasaponin XLIX
Catalog No.:BCN8470
CAS No.:1033593-12-2
- Physalin G
Catalog No.:BCN7815
CAS No.:76045-38-0
- Guvacine
Catalog No.:BCN6529
CAS No.:498-96-4
- Luteolin-4'-O-glucoside
Catalog No.:BCN8734
CAS No.:6920-38-3
- 1,3,6-Trihydroxy-2-methylanthraquinone 3-O-(6'-O-acetyl)-alpha-L-rhamnosyl-(1->2)-Beta-D-glucoside
Catalog No.:BCN6384
CAS No.:87686-87-1
- Zerumbone
Catalog No.:BCN6363
CAS No.:471-05-6
- Xanthoangelol F
Catalog No.:BCN8324
CAS No.:265652-71-9
- 6''-O-acetylsaikosaponin A
Catalog No.:BCN6478
CAS No.:64340-46-1
- Kaempferol 3-O-beta-(6''-p-coumaroyl)glucopyranosyl(1->2)-alpha-L-rhamnopyranoside
Catalog No.:BCN7711
CAS No.:111957-48-3
- Orcinol 1-O-beta-D-apiofuranosyl-(1->6)-beta-D-glucopyranoside
Catalog No.:BCN8273
CAS No.:868557-54-4
- Luteolin 7-rutinoside
Catalog No.:BCN6360
CAS No.:20633-84-5
- 3'-Angeloyloxy-4'-senecioyloxy-2',3'-dihydrooroselol
Catalog No.:BCN8730
CAS No.:1221686-60-7
- Cyclo(Hyp-Val)
Catalog No.:BCN6391
CAS No.:1425501-89-8
- 3'-Methoxymirificin
Catalog No.:BCN6377
CAS No.:1297609-29-0
- 2-Cinnamoyl-1-galloylglucose
Catalog No.:BCN6383
CAS No.:56994-83-3
- Quinizarin monoglucoside
Catalog No.:BCN6382
CAS No.:39115-11-2
- Cassiaside C
Catalog No.:BCN8733
CAS No.:119170-52-4
- Armepavine
Catalog No.:BCN8490
CAS No.:524-20-9
Cytotoxic constituents from Helicteres hirsuta collected in Vietnam.[Pubmed:30445838]
Nat Prod Res. 2018 Nov 16:1-5.
Phytochemical study on the extract of Vietnamese medicinal plant Helicteres hirsuta Lour. has led to the isolation and structural elucidation of twelve secondary metabolites, 3-O-trans-caffeoylbetulinic acid (1), 3beta-benzoylbetulinic acid (2), Betulinic acid methyl ester (3), betulinic acid (4), lupeol (5), 4-hydroxybenzoic acid (6), 3,4-dihydroxybenzoic acid methyl ester (7), 4-hydroxy-3,5-dimethoxybenzoic acid (8), 5,8-dihydroxy-7,4'-dimethoxyflavone (9), isoscutellarein 4'-methyl ether 8-O-beta-D-glucopyranoside (10), methyl caffeate (11) and stigmasterol (12). Especially, compound 2 was reported as a new natural product. Their structures were elucidated by a combination of 2D NMR and ESI-FT-ICR-MS spectroscopies. Furthermore, eight compounds were tested for their cytotoxicity against five cancer cell lines (Hela, HepG2, SK-LU-1, AGS and SK-MEL-2). The results showed that compounds (1, 3-5, 9) have moderate activities. This is the first study on the chemical constituents and their cytotoxicity of the Vietnamese Helicteres hirsuta L.
Synthesis of cytotoxically active derivatives based on alkylated 2,3-seco-triterpenoids.[Pubmed:28923388]
Eur J Med Chem. 2017 Nov 10;140:74-83.
Extremely low content of biologically active triterpenoids with the fragmented or contracted ring A extractable from plants is the main disadvantage of their use in drug discovery and practical pharmacology. Development of new methods for synthesis of these compounds and their structural analogs from bioavailable triterpene precursors gives an opportunity to obtain promising agents for pharmacology with excellent yields. A new approach to synthesis of alkylated A-seco-triterpenoids, including the Beckmann fragmentation of 3-methyl-substituted allobetulin or Betulinic acid methyl ester with 2-hydroxyimino group in the ring A was proposed. These compounds were used to prepare a series of 2,3-seco- and five-membered ring A lupane and oleanane derivatives, cytotoxicity of which was screened in vitro against the cancer (HEp-2, HCT 116, A549, RD TE32, MS) and non-cancerous (HEK 293) cell lines. Methyl 3-bromomethyl-1-cyano-3-oxo-2,3-seco-2-norlup-20(29)-en-30-al-28-oate was selected as the most active compound (IC50 3.4-10.4 muM for HEp-2, HCT 116, RD TE32, MS cells) capable of triggering caspase-8-mediated apoptosis in HCT 116 cells accompanied by typical apoptotic chromatin condensation, without any loss of mitochondrial membrane permeability.
Antiprotozoal activity of betulinic acid derivatives.[Pubmed:19748254]
Phytomedicine. 2010 Apr;17(5):379-82.
Betulinic acid (1), isolated from the crude extract of the leaves of Pentalinon andrieuxii (Apocynaceae), together with betulinic acid acetate (2), betulonic acid (3), Betulinic acid methyl ester (4), and betulin (5) were evaluated for their antiprotozoal activity. The results showed that modifying the C-3 position increases leishmanicidal activity while modification of the C-3 and C-28 positions decreases trypanocidal activity.
A new dihydrodibenzodioxinone from Hypericum x 'Hidcote'.[Pubmed:19535015]
Fitoterapia. 2009 Jun;80(4):226-9.
One new compound, 4-hydroxy-4a,7-dimethoxy-4,4a-dihydrodibenzo-p-dioxin-2(3H)-one (1), was isolated from the aerial parts of the hybrid Hypericum x 'Hidcote', together with 8 known compounds: caryophyllene-4,5-epoxide, quercetin, quercitrin, quercetin-3-O-beta-D-galactopyranoside, epicatechin, Betulinic acid methyl ester, beta-sitosterol and beta-sitosterol glucoside. The structure of the new compound, as well as its absolute configuration, was established by means of spectroscopic data analyses, including 2D NMR spectroscopy and X-ray structural analysis.
Antimalarial activity of betulinic acid and derivatives in vitro against Plasmodium falciparum and in vivo in P. berghei-infected mice.[Pubmed:19367418]
Parasitol Res. 2009 Jul;105(1):275-9.
Malaria is one of the most important tropical diseases and mainly affects populations living in developing countries. Reduced sensitivity of Plasmodium sp. to formerly recommended antimalarial drugs places an increasing burden on malaria control programs as well as on national health systems in endemic countries. The present study aims to evaluate the antimalarial activity of betulinic acid and its derivative compounds, betulonic acid, betulinic acid acetate, Betulinic acid methyl ester, and Betulinic acid methyl ester acetate. These substances showed antiplasmodial activity against chloroquine-resistant Plasmodium falciparum parasites in vitro, with IC(50) values of 9.89, 10.01, 5.99, 51.58, and 45.79 microM, respectively. Mice infected with Plasmodium berghei and treated with betulinic acid acetate had a dose-dependent reduction of parasitemia. Our results indicate that betulinic acid and its derivative compounds are candidates for the development of new antimalarial drugs.
Isolation of betulinic acid, its methyl ester and guaiane sesquiterpenoids with protein tyrosine phosphatase 1B inhibitory activity from the roots of Saussurea lappa C.B.Clarke.[Pubmed:19136914]
Molecules. 2009 Jan 8;14(1):266-72.
Activity-guided fractionation of a MeOH extract of the roots of Saussurea lappa C.B.Clarke (Compositae), using an in vitro protein tyrosine phosphatase 1B (PTP1B) inhibition assay, led to the isolation of four active constituents: betulinic acid (1), Betulinic acid methyl ester (2), mokko lactone (3) and dehydrocostuslactone (4), along with nine inactive compounds. Our findings indicate that betulinic acid (1) and its methyl ester 2, as well as the two guaiane sesquiterpenoids 3 and 4 are potential lead moieties for the development of new PTP1B inhibitors.
Melanogenesis inhibitory compounds from Saussureae Radix.[Pubmed:18409040]
Arch Pharm Res. 2008 Mar;31(3):294-9.
Ten compounds were isolated from the EtOAc soluble part of the MeOH extract of Saussureae Radix, with their effects on melanin production also evaluated in B-16 mouse melanoma cell lines stimulated with 3-isobutyl-1-methylxanthine (IBMX), an elevator of cellular cAMP. The compounds were identified as aplotaxene (1), 1 beta-hydroxy arbusculin A (2), costunolide (3), dehydrocostuslactone (4), 11 beta,13-dihydrocostunolide (5), reynosin (6), heptadec-(9Z)-enoic acid (7), beta-sitosterol (8), linoleic acid methyl ester (9) and Betulinic acid methyl ester (10). Compounds 2, 9 and 10 were identified from Saussureae Radix for the first time. Furthermore, compounds 2, 3 and 6 showed potent inhibitory effects on the IBMX-induced melanogenesis, in dose-dependent manners, with IC50 values of 11, 3 and 2.5 microg/mL, respectively. As a positive control, arbutin exhibited an IC50 value of 29 microg/mL.
Lanostane-type triterpenoids from Diospyros discolor.[Pubmed:17541192]
Chem Pharm Bull (Tokyo). 2007 Jun;55(6):908-11.
Four new lanostane-type triterpenes, 24-ethyl-3beta-methoxylanost-9(11)-en-25-ol (1), 3beta-methoxy-24-methylenelanost-9(11)-en-25-ol (2), 3beta-methoxy-25-methyl-24-methylenelanost-9(11)-en-21-ol (3) and 3beta-methoxy-24-methyllanosta-9(11),25-dien-24-ol (4) together with three known triterpenes, betulinaldehyde, Betulinic acid methyl ester, and ursaldehyde have been isolated from the methanol extract of the twigs of Diospyros discolor. The structures of those new compounds were elucidated by spectroscopic methods.


