ArmepavineCAS# 524-20-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 524-20-9 | SDF | Download SDF |
| PubChem ID | 442169 | Appearance | White powder |
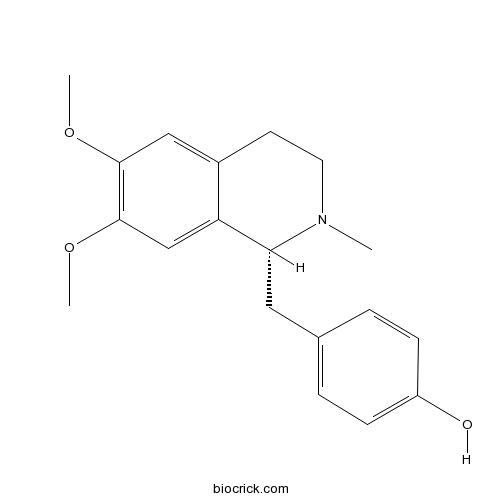
| Formula | C19H23NO3 | M.Wt | 313.39 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Alkaloid D from Evonymus europaea; Evoeuropine | ||
| Solubility | Soluble in methanol and chloroform | ||
| Chemical Name | 4-[[(1~{R})-6,7-dimethoxy-2-methyl-3,4-dihydro-1~{H}-isoquinolin-1-yl]methyl]phenol | ||
| SMILES | CN1CCC2=CC(=C(C=C2C1CC3=CC=C(C=C3)O)OC)OC | ||
| Standard InChIKey | ZBKFZIUKXTWQTP-QGZVFWFLSA-N | ||
| Standard InChI | InChI=1S/C19H23NO3/c1-20-9-8-14-11-18(22-2)19(23-3)12-16(14)17(20)10-13-4-6-15(21)7-5-13/h4-7,11-12,17,21H,8-10H2,1-3H3/t17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Armepavine exerts both in vitro and in vivo antifibrotic effects in rats, with inhibition of NF-κB, JunD and C/EBPß pathways. It also shows cytotoxic activity. |
| Targets | NF-κB | JunD | C/EBPß |
| In vitro | Phytochemical study and cytotoxic activity of alkaloids from Uvaria chamae P. Beauv.[Pubmed: 11031775]Pharmazie. 2000 Sep;55(9):688-9.
|
| In vivo | Effects of armepavine against hepatic fibrosis induced by thioacetamide in rats.[Pubmed: 21717514 ]Phytother Res. 2012 Mar;26(3):344-53.The aim of this study was to investigate if Armepavine (Arm, C₁₉H₂₃O₃N) could exert inhibitory effects against hepatic fibrosis in rats.
|
| Structure Identification | Molecules. 2016 Jul 19;21(7).Quantitative Determination of Alkaloids in Lotus Flower (Flower Buds of Nelumbo nucifera) and Their Melanogenesis Inhibitory Activity.[Reference: WebLink]A quantitative analytical method for five aporphine alkaloids, nuciferine (1), nornuciferine (2), N-methylasimilobine (3), asimilobine (4), and pronuciferine (5), and five benzylisoquinoline alkaloids, Armepavine (6), norArmepavine (7), N-methylcoclaurine (8), coclaurine (9), and norjuziphine (10), identified as the constituents responsible for the melanogenesis inhibitory activity of the extracts of lotus flowers (the flower buds of Nelumbo nucifera), has been developed using liquid chromatography-mass spectrometry. |

Armepavine Dilution Calculator

Armepavine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1909 mL | 15.9546 mL | 31.9091 mL | 63.8182 mL | 79.7728 mL |
| 5 mM | 0.6382 mL | 3.1909 mL | 6.3818 mL | 12.7636 mL | 15.9546 mL |
| 10 mM | 0.3191 mL | 1.5955 mL | 3.1909 mL | 6.3818 mL | 7.9773 mL |
| 50 mM | 0.0638 mL | 0.3191 mL | 0.6382 mL | 1.2764 mL | 1.5955 mL |
| 100 mM | 0.0319 mL | 0.1595 mL | 0.3191 mL | 0.6382 mL | 0.7977 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cassiaside C
Catalog No.:BCN8733
CAS No.:119170-52-4
- Quinizarin monoglucoside
Catalog No.:BCN6382
CAS No.:39115-11-2
- 2-Cinnamoyl-1-galloylglucose
Catalog No.:BCN6383
CAS No.:56994-83-3
- 3'-Methoxymirificin
Catalog No.:BCN6377
CAS No.:1297609-29-0
- Cyclo(Hyp-Val)
Catalog No.:BCN6391
CAS No.:1425501-89-8
- 3'-Angeloyloxy-4'-senecioyloxy-2',3'-dihydrooroselol
Catalog No.:BCN8730
CAS No.:1221686-60-7
- Luteolin 7-rutinoside
Catalog No.:BCN6360
CAS No.:20633-84-5
- Orcinol 1-O-beta-D-apiofuranosyl-(1->6)-beta-D-glucopyranoside
Catalog No.:BCN8273
CAS No.:868557-54-4
- Kaempferol 3-O-beta-(6''-p-coumaroyl)glucopyranosyl(1->2)-alpha-L-rhamnopyranoside
Catalog No.:BCN7711
CAS No.:111957-48-3
- 6''-O-acetylsaikosaponin A
Catalog No.:BCN6478
CAS No.:64340-46-1
- Xanthoangelol F
Catalog No.:BCN8324
CAS No.:265652-71-9
- Betulinic acid methyl ester
Catalog No.:BCN6378
CAS No.:2259-06-5
- Suavissimoside F1
Catalog No.:BCN6361
CAS No.:95645-51-5
- Trillenoside A
Catalog No.:BCN6387
CAS No.:58809-09-9
- Dehydrodicentrine
Catalog No.:BCN6364
CAS No.:19843-03-9
- Deoxytrillenoside A
Catalog No.:BCN6376
CAS No.:77658-50-5
- Isorubrofusarin 10-gentiobioside
Catalog No.:BCN7202
CAS No.:200127-93-1
- Rhamnetin 3-galactoside
Catalog No.:BCN6358
CAS No.:62858-07-5
- 6-O-(E)-Caffeoylglucopyranose
Catalog No.:BCN6393
CAS No.:209797-79-5
- 1,4-Dihydroxy-2-carbomethoxy-3-prenylnaphthalene-1-O-beta-glucopyranoside
Catalog No.:BCN6375
CAS No.:1415729-43-9
- 4-[4-[[6-O-[(2E)-3-(4-Hydroxyphenyl)-1-oxo-2-propen-1-yl]-2-O-(3,4,5-trihydroxybenzoyl)-beta-D-glucopyranosyl]oxy]phenyl]-2-butanone
Catalog No.:BCN6359
CAS No.:105274-16-6
- Soyasapogenol D
Catalog No.:BCN6370
CAS No.:65892-76-4
- cis-1-o-Caffeoylquinic acid
Catalog No.:BCN6390
CAS No.:1627537-95-4
- Macrophylloside D
Catalog No.:BCN6366
CAS No.:179457-69-3
Inhibition of (S)-armepavine from Nelumbo nucifera on autoimmune disease of MRL/MpJ-lpr/lpr mice.[Pubmed:16413531]
Eur J Pharmacol. 2006 Feb 15;531(1-3):270-9.
T cell immune responses play important roles in the pathogenesis of systemic lupus erythematosus (SLE). (S)-Armepavine (C19H23O3N; MW313) from Nelumbo nucifera suppresses T cells proliferation. To study its potential benefit on SLE, we examined effects of (S)-Armepavine on MRL/MpJ-lpr/lpr mice, which have similar disease features to human SLE. MRL/MpJ-lpr/lpr mice were treated orally with (S)-Armepavine for 6 weeks and their SLE characteristics were evaluated. The results revealed that (S)-Armepavine prevented lymphadenopathy and elongated life span of MRL/MpJ-lpr/lpr mice. It seemed to be mediated by inhibition of splenocytes proliferation, suppression of interleukin-2 (IL-2), interleukin-4, interleukin-10, and interferon-gamma (IFN-gamma) gene expressions, reduction of glomerular hypercellularity and immune complexes deposition, and decrease of urinary protein and anti-double stranded DNA autoantibody production. Furthermore, the data demonstrated (S)-Armepavine impaired IL-2 and IFN-gamma transcripts in human peripheral blood mononuclear cells. We suggest that (S)-Armepavine may be an immunomodulator for the management of autoimmune diseases like SLE.


