2,6-Dimethoxy-1,4-benzoquinoneCAS# 530-55-2 |
Quality Control & MSDS
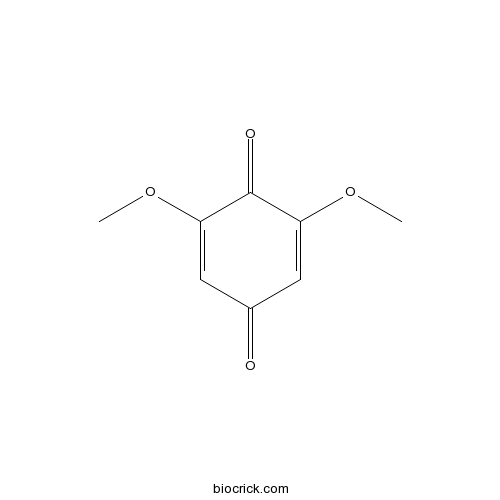
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 530-55-2 | SDF | Download SDF |
| PubChem ID | 68262 | Appearance | Yellow powder |
| Formula | C8H8O4 | M.Wt | 168.2 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,6-dimethoxycyclohexa-2,5-diene-1,4-dione | ||
| SMILES | COC1=CC(=O)C=C(C1=O)OC | ||
| Standard InChIKey | OLBNOBQOQZRLMP-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 2,6-Dimethoxy-1,4-benzoquinone exhibits strong antibacterial activities against S. pyogenes, S. mitis, and S. mutans with MIC values of 7.8, 7.8, and 15.6 ug/mL, and MBC values of 7.8, 7.8, and 31.2 ug/mL, respectively. 2,6-Dimethoxy-1,4-benzoquinone can produce genotoxic effects in patients taking dimethophrine. |
| Targets | Antifection |
| In vitro | Antimicrobial activities of some Thai traditional medical longevity formulations from plants and antibacterial compounds from Ficus foveolata.[Pubmed: 24611777]Pharm Biol. 2014 Sep;52(9):1104-9.Medicinal plants involved in traditional Thai longevity formulations are potential sources of antimicrobial compounds.
To evaluate the antimicrobial activities of some extracts from medicinal plants used in traditional Thai longevity formulations against some oral pathogens, including Streptococcus pyogenes, Streptococcus mitis, Streptococcus mutans, and Candida albicans. An extract that possessed the strongest antimicrobial activity was fractionated to isolate and identify the active compounds.
Formation of 2,6-dimethoxy-1,4-benzoquinone, a highly genotoxic compound, from the reaction of sodium nitrite with the sympathomimetic drug dimethophrine in acidic aqueous solution.[Pubmed: 3208895]Farmaco Sci. 1988 Jun;43(6):523-38.Because of the genotoxic effects shown by Dimethophrine (DMP) nitrosation mixtures, the interaction between DMP hydrochloride and sodium nitrite in acidic aqueous solution at 37 degrees was investigated in a wide range of reagent concentrations and molar ratios, reaction times and pH values. |
| In vivo | Cytotoxic, DNA-damaging and mutagenic properties of 2,6-dimethoxy-1,4-benzoquinone, formed by dimethophrine-nitrite interaction.[Pubmed: 3252018]J Pharmacol Exp Ther. 1988 Mar;244(3):1011-5.In conditions similar to those occurring in the stomach, the sympathomimetic drug dimethophrine was found to react with nitrite yielding 2,6-Dimethoxy-1,4-benzoquinone (DMBQ). |

2,6-Dimethoxy-1,4-benzoquinone Dilution Calculator

2,6-Dimethoxy-1,4-benzoquinone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.9453 mL | 29.7265 mL | 59.453 mL | 118.9061 mL | 148.6326 mL |
| 5 mM | 1.1891 mL | 5.9453 mL | 11.8906 mL | 23.7812 mL | 29.7265 mL |
| 10 mM | 0.5945 mL | 2.9727 mL | 5.9453 mL | 11.8906 mL | 14.8633 mL |
| 50 mM | 0.1189 mL | 0.5945 mL | 1.1891 mL | 2.3781 mL | 2.9727 mL |
| 100 mM | 0.0595 mL | 0.2973 mL | 0.5945 mL | 1.1891 mL | 1.4863 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Deoxyvasicinone
Catalog No.:BCN5697
CAS No.:530-53-0
- L-Picein
Catalog No.:BCC8336
CAS No.:530-14-3
- Indomethacin
Catalog No.:BCC3794
CAS No.:53-86-1
- Dehydroepiandrosterone
Catalog No.:BCN2202
CAS No.:53-43-0
- Oxandrolone
Catalog No.:BCC5242
CAS No.:53-39-4
- Methylprednisolone acetate
Catalog No.:BCC9043
CAS No.:53-36-1
- Cocaine hydrochloride
Catalog No.:BCC5943
CAS No.:53-21-4
- Mitotane (Lsodren)
Catalog No.:BCC3815
CAS No.:53-19-0
- Estrone
Catalog No.:BCN2201
CAS No.:53-16-7
- Prednisone
Catalog No.:BCC4957
CAS No.:53-03-2
- Acetylisocupressic acid
Catalog No.:BCN5695
CAS No.:52992-82-2
- Ajugol
Catalog No.:BCN2883
CAS No.:52949-83-4
- Syringic acid
Catalog No.:BCN5699
CAS No.:530-57-4
- Sinapic acid
Catalog No.:BCN3539
CAS No.:530-59-6
- CDI (1,1′-Carbonyldiimidazole)
Catalog No.:BCC2809
CAS No.:530-62-1
- Salinomycin
Catalog No.:BCC1916
CAS No.:53003-10-4
- Murralongin
Catalog No.:BCN5696
CAS No.:53011-72-6
- T-5224
Catalog No.:BCC5383
CAS No.:530141-72-1
- Scutebarbatine J
Catalog No.:BCN8134
CAS No.:960302-85-6
- Morellic acid
Catalog No.:BCN3073
CAS No.:5304-71-2
- 9,13-Epidioxy-8(14)-abieten-18-oic acid
Catalog No.:BCN1426
CAS No.:5309-35-3
- Dichotomin
Catalog No.:BCN2836
CAS No.:53093-47-3
- Androsin
Catalog No.:BCN3842
CAS No.:531-28-2
- Coniferin
Catalog No.:BCN5700
CAS No.:531-29-3
Cytotoxic, DNA-damaging and mutagenic properties of 2,6-dimethoxy-1,4-benzoquinone, formed by dimethophrine-nitrite interaction.[Pubmed:3252018]
J Pharmacol Exp Ther. 1988 Mar;244(3):1011-5.
In conditions similar to those occurring in the stomach, the sympathomimetic drug dimethophrine was found to react with nitrite yielding 2,6-Dimethoxy-1,4-benzoquinone (DMBQ). The in vitro and in vivo studies carried out to evaluate the capability of DMBQ to produce cytotoxic and genotoxic effects provided the following results. A dose-related reduction of V79 cells plating efficiency was observed for DMBQ concentrations ranging from 10 to 80 microM; a similar reduction in the fraction of viable cells excluding trypan blue occurred after exposure to 4-fold higher concentrations. A dose-dependent amount of DNA fragmentation was revealed by the alkaline elution technique either in V79 cells exposed to DMBQ concentrations ranging from 10 to 80 microM or in kidney, gastric mucosa and brain of rats treated with single p.o. doses ranging from 33 to 300 mg/kg. Both in vitro and in vivo DNA lesions were largely repaired within 24 hr, but their promutagenic character was demonstrated by the induction of 6-thioguanine-resistance in V79 cells. Primary cultures of rat hepatocytes displayed a greater resistance to the cytotoxic and DNA-damaging activities of DMBQ, and did not exhibit a clear evidence of DNA repair synthesis. Similarly, DNA fragmentation was practically undetectable in the rat liver. Therefore, DMBQ should be considered as a direct-acting genotoxic chemical which is metabolized to less, or nonreactive, species. These findings suggest that DMBQ could produce genotoxic effects in patients taking dimethophrine.
Antimicrobial activities of some Thai traditional medical longevity formulations from plants and antibacterial compounds from Ficus foveolata.[Pubmed:24611777]
Pharm Biol. 2014 Sep;52(9):1104-9.
CONTEXT: Medicinal plants involved in traditional Thai longevity formulations are potential sources of antimicrobial compounds. OBJECTIVE: To evaluate the antimicrobial activities of some extracts from medicinal plants used in traditional Thai longevity formulations against some oral pathogens, including Streptococcus pyogenes, Streptococcus mitis, Streptococcus mutans, and Candida albicans. An extract that possessed the strongest antimicrobial activity was fractionated to isolate and identify the active compounds. MATERIALS AND METHODS: Methanol and ethyl acetate extracts of 25 medicinal plants used as Thai longevity formulations were evaluated for their antimicrobial activity using disc diffusion (5 mg/disc) and broth microdilution (1.2-2500 microg/mL) methods. The ethyl acetate extract of Ficus foveolata Wall. (Moraceae) stems that exhibited the strongest antibacterial activity was fractionated to isolate the active compounds by an antibacterial assay-guided isolation process. RESULTS AND DISCUSSION: The ethyl acetate extract of F. foveolata showed the strongest antibacterial activity with minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values of 19.5-39.0 and 39.0-156.2 microg/mL, respectively. On the basis of an antibacterial assay-guided isolation, seven antibacterial compounds, including 2,6-Dimethoxy-1,4-benzoquinone (1), syringaldehyde (2), sinapaldehyde (3), coniferaldehyde (4), 3beta-hydroxystigmast-5-en-7-one (5), umbelliferone (6), and scopoletin (7), were purified. Among these isolated compounds, 2,6-Dimethoxy-1,4-benzoquinone (1) exhibited the strongest antibacterial activities against S. pyogenes, S. mitis, and S. mutans with MIC values of 7.8, 7.8, and 15.6 microg/mL, and MBC values of 7.8, 7.8, and 31.2 microg/mL, respectively. In addition, this is the first report of these antibacterial compounds in the stems of F. foveolata.


