DehydrocavidineCAS# 83218-34-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83218-34-2 | SDF | Download SDF |
| PubChem ID | 92043552 | Appearance | Powder |
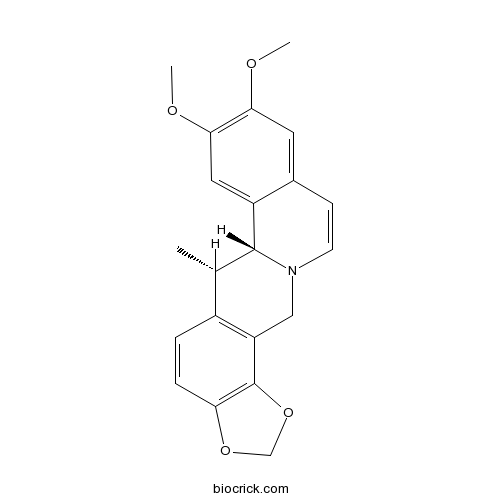
| Formula | C21H21NO4 | M.Wt | 351.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (12S,13R)-16,17-dimethoxy-12-methyl-5,7-dioxa-1-azapentacyclo[11.8.0.03,11.04,8.014,19]henicosa-3(11),4(8),9,14,16,18,20-heptaene | ||
| SMILES | CC1C2C3=CC(=C(C=C3C=CN2CC4=C1C=CC5=C4OCO5)OC)OC | ||
| Standard InChIKey | XSOKSDXTNIQQJD-FKIZINRSSA-N | ||
| Standard InChI | InChI=1S/C21H21NO4/c1-12-14-4-5-17-21(26-11-25-17)16(14)10-22-7-6-13-8-18(23-2)19(24-3)9-15(13)20(12)22/h4-9,12,20H,10-11H2,1-3H3/t12-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Dehydrocorydaline has antitumor activity. 2. Dehydrocorydaline inhibits MCF-7 cell proliferation by inducing apoptosis mediated by regulating Bax/Bcl-2, activating caspases as well as cleaving PARP. 3. Dehydrocorydaline reduces the viability of macrophage-derived RAW264.7 cells and primary macrophages in the presence of LPS. 4. Dehydrocorydaline inhibits the elevation of mitochondrial membrane potential and induces ATP depletion in LPS-stimulated macrophages but neither affects basal mitochondrial membrane potential nor ATP content in non-stimulated macrophages. |
| Targets | Bcl-2/Bax | Caspase | IL Receptor | PARP | cAMP | PGE |

Dehydrocavidine Dilution Calculator

Dehydrocavidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8458 mL | 14.2288 mL | 28.4576 mL | 56.9152 mL | 71.144 mL |
| 5 mM | 0.5692 mL | 2.8458 mL | 5.6915 mL | 11.383 mL | 14.2288 mL |
| 10 mM | 0.2846 mL | 1.4229 mL | 2.8458 mL | 5.6915 mL | 7.1144 mL |
| 50 mM | 0.0569 mL | 0.2846 mL | 0.5692 mL | 1.1383 mL | 1.4229 mL |
| 100 mM | 0.0285 mL | 0.1423 mL | 0.2846 mL | 0.5692 mL | 0.7114 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1beta-Hydroxy-beta-eudesmol
Catalog No.:BCN7097
CAS No.:83217-89-4
- ISX 9
Catalog No.:BCC6181
CAS No.:832115-62-5
- Astragaloside A
Catalog No.:BCC6494
CAS No.:83207-58-3
- 2',4',6'-Trimethoxyacetophenone
Catalog No.:BCN4847
CAS No.:832-58-6
- Angelol G
Catalog No.:BCN7963
CAS No.:83199-38-6
- 8alpha-Tigloyloxyhirsutinolide 13-O-acetate
Catalog No.:BCN7108
CAS No.:83182-58-5
- Kaempferol 3-O-rhamninoside
Catalog No.:BCN6845
CAS No.:83170-31-4
- Bonducellin
Catalog No.:BCN6823
CAS No.:83162-84-9
- Angelol B
Catalog No.:BCN8037
CAS No.:83156-04-1
- Carpalasionin
Catalog No.:BCN7644
CAS No.:83150-97-4
- Octreotide acetate
Catalog No.:BCC5643
CAS No.:83150-76-9
- (+)-Sophoridine
Catalog No.:BCC8360
CAS No.:83148-91-8
- Angelol H
Catalog No.:BCN8047
CAS No.:83247-73-8
- APD668
Catalog No.:BCC5389
CAS No.:832714-46-2
- LF 11
Catalog No.:BCC6174
CAS No.:832729-13-2
- Lauryl-LF 11
Catalog No.:BCC6175
CAS No.:832729-14-3
- Napabucasin
Catalog No.:BCC6525
CAS No.:83280-65-3
- Menisporphine
Catalog No.:BCN7902
CAS No.:83287-02-9
- 7-Hydroxycoumarin-6-carboxylic acid
Catalog No.:BCN4375
CAS No.:833-52-3
- Bryostatin 1
Catalog No.:BCC7343
CAS No.:83314-01-6
- 8(17),13-Labdadien-15,16-olide
Catalog No.:BCN4374
CAS No.:83324-51-0
- (-)-Lariciresinol
Catalog No.:BCN3418
CAS No.:83327-19-9
- Dynorphin B
Catalog No.:BCC5987
CAS No.:83335-41-5
- 10-O-Caffeoyl-6-epiferetoside
Catalog No.:BCN4822
CAS No.:83348-22-5
Selective binding and highly sensitive fluorescent sensor of palmatine and dehydrocorydaline alkaloids by cucurbit[7]uril.[Pubmed:19532985]
Org Biomol Chem. 2009 Jul 7;7(13):2699-703.
The complexation behavior of palmatine (P) and dehydrocorydaline (DHC) alkaloid guest molecules by cucurbit[7]uril (CB7) host have been investigated by means of fluorescence spectra in aqueous phosphate buffer solution (pH 7.2). It is found that each alkaloid exhibits dramatic fluorescence enhancement upon complexation with CB7, and the intensity of the emittance is strong enough to be readily distinguished by the naked eye. Although the two guests possess similar structure, the complex stability constant of P with CB7 is 5.4 times larger than that of DHC. 1H NMR studies show that the binding modes differ much, i.e., deep encapsulation for P-CB7 and shallow encapsulation for DHC-CB7. Furthermore, the solvent effects and salt effects during the course of complexation have also been investigated, showing they significantly influence the binding ability and selectivity of CB7 with the alkaloid guests. Particularly, addition of a small amount (4 vol%) of ethanol increases the P/DHC selectivity to 17.2.
Dehydrocorydaline inhibits elevated mitochondrial membrane potential in lipopolysaccharide-stimulated macrophages.[Pubmed:21575743]
Int Immunopharmacol. 2011 Sep;11(9):1362-7.
Activated macrophages play a critical role in the pathogenesis of numerous diseases by producing pro-inflammatory cytokines such as interleukin (IL)-1beta and IL-6. While the mechanisms of bacterial component recognition and signal transduction have been well investigated, viability regulation in activated macrophages remains unclear. We screened herbal ingredients to find an agent that reduces the viability of lipopolysaccharide (LPS)-stimulated macrophages and observed that dehydrocorydaline, a component of Corydalis yanhusuo, reduced the viability of macrophage-derived RAW264.7 cells and primary macrophages in the presence of LPS. Dehydrocorydaline inhibited the elevation of mitochondrial membrane potential and induced ATP depletion in LPS-stimulated macrophages but neither affected basal mitochondrial membrane potential nor ATP content in non-stimulated macrophages. Dehydrocorydaline also prevented increased concentrations of IL-1beta and IL-6 in culture media of LPS-stimulated macrophages. Mode of dehydrocorydaline action indicates that elevated mitochondrial membrane potential may be a novel target to specifically reduce viability and suppress cytokine production in LPS-stimulated macrophages.
Effect of wine and vinegar processing of Rhizoma Corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats.[Pubmed:22258341]
Molecules. 2012 Jan 18;17(1):951-70.
Vinegar and wine processing of medicinal plants are two traditional pharmaceutical techniques which have been used for thousands of years in China. Tetrahydropalmatine (THP), dehydrocorydaline (DHC) and protopine are three major bioactive molecules in Rhizoma Corydalis. In this study, a simple and reliable HPLC method was developed for simultaneous analysis of THP, DHC and protopine in rat tissues after gastric gavage administration of Rhizoma Corydalis. The validated HPLC method was successfully applied to investigate the effect of wine and vinegar processing on the compounds' distribution in rat tissues. Our results showed that processing mainly affect the T(max) and mean residence time (MRT) of the molecules without changing their C(max) and AUC(0-24)( )(h) Vinegar processing significantly increased the T(max) of DHC in heart, kidney, cerebrum, cerebrellum, brain stem and striatum and prolonged the T(max) of protopine in brain. No significant changes were observed on the T(max) of THP in rat tissues after vinegar processing. Wine processing reduced the T(max) of protopine and DHC in liver and spleen and T(max) of protopine in lung, but increased the T(max) of THP in all the rat tissues examined. To our knowledge, this is the first report on the effects of processing on the tissue distribution of the bioactive molecules from Rhizoma Corydalis.


