DSCCAS# 74124-79-1 |
- Valproic acid sodium salt (Sodium valproate)
Catalog No.:BCC2156
CAS No.:1069-66-5
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- Panobinostat (LBH589)
Catalog No.:BCC3601
CAS No.:404950-80-7
- Belinostat (PXD101)
Catalog No.:BCC2153
CAS No.:414864-00-9
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 74124-79-1 | SDF | Download SDF |
| PubChem ID | 676246 | Appearance | Powder |
| Formula | C9H8N2O7 | M.Wt | 256.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
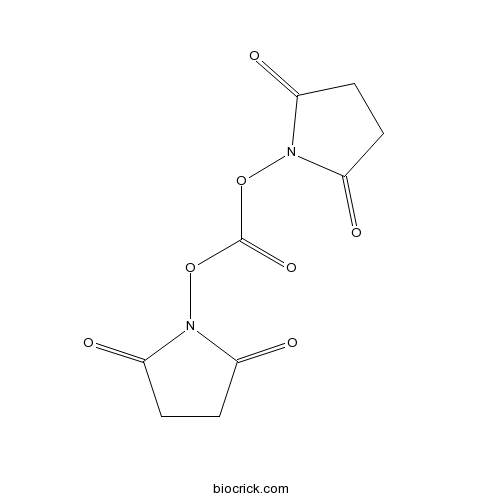
| Chemical Name | bis(2,5-dioxopyrrolidin-1-yl) carbonate | ||
| SMILES | C1CC(=O)N(C1=O)OC(=O)ON2C(=O)CCC2=O | ||
| Standard InChIKey | PFYXSUNOLOJMDX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H8N2O7/c12-5-1-2-6(13)10(5)17-9(16)18-11-7(14)3-4-8(11)15/h1-4H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

DSC Dilution Calculator

DSC Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9032 mL | 19.516 mL | 39.032 mL | 78.064 mL | 97.58 mL |
| 5 mM | 0.7806 mL | 3.9032 mL | 7.8064 mL | 15.6128 mL | 19.516 mL |
| 10 mM | 0.3903 mL | 1.9516 mL | 3.9032 mL | 7.8064 mL | 9.758 mL |
| 50 mM | 0.0781 mL | 0.3903 mL | 0.7806 mL | 1.5613 mL | 1.9516 mL |
| 100 mM | 0.039 mL | 0.1952 mL | 0.3903 mL | 0.7806 mL | 0.9758 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
DSC
- Norjuziphine
Catalog No.:BCN3367
CAS No.:74119-87-2
- SKF 83822 hydrobromide
Catalog No.:BCC7252
CAS No.:74115-10-9
- Ketorolac tromethamine salt
Catalog No.:BCC4431
CAS No.:74103-07-4
- Ketorolac
Catalog No.:BCC5190
CAS No.:74103-06-3
- ACV 1
Catalog No.:BCC5989
CAS No.:740980-24-9
- Cudratricusxanthone A
Catalog No.:BCN7649
CAS No.:740810-42-8
- Macamide B
Catalog No.:BCN1366
CAS No.:74058-71-2
- Ketanserin
Catalog No.:BCC5050
CAS No.:74050-98-9
- Lancifolin C
Catalog No.:BCN2019
CAS No.:74048-71-8
- Enoxacin (Penetrex)
Catalog No.:BCC3775
CAS No.:74011-58-8
- p-Hydroxy-cinnamic acid
Catalog No.:BCN5027
CAS No.:7400-08-0
- Mosloflavone
Catalog No.:BCN6796
CAS No.:740-33-0
- Bisdethiobis(methylthio)gliotoxin
Catalog No.:BCN7351
CAS No.:74149-38-5
- Pimobendan
Catalog No.:BCC2294
CAS No.:74150-27-9
- 2,3-Dehydrokievitone
Catalog No.:BCN4294
CAS No.:74161-25-4
- R547
Catalog No.:BCC3927
CAS No.:741713-40-6
- Haginin A
Catalog No.:BCN6861
CAS No.:74174-29-1
- Cyclokievitone
Catalog No.:BCC8159
CAS No.:74175-82-9
- Doxazosin
Catalog No.:BCC4218
CAS No.:74191-85-8
- Uplandicine
Catalog No.:BCN2055
CAS No.:74202-10-1
- 3-Bromo-7-nitroindazole
Catalog No.:BCC6770
CAS No.:74209-34-0
- Vintafolide
Catalog No.:BCC5265
CAS No.:742092-03-1
- Carbenoxolone disodium
Catalog No.:BCC3745
CAS No.:7421-40-1
- OSU-03012 (AR-12)
Catalog No.:BCC1255
CAS No.:742112-33-0
FTIR, XRD and DSC studies of nanochitosan, cellulose acetate and polyethylene glycol blend ultrafiltration membranes.[Pubmed:28363652]
Int J Biol Macromol. 2017 Nov;104(Pt B):1721-1729.
In the present work, a series of novel nanochitosan/cellulose acetate/polyethylene glycol (NCS/CA/PEG) blend flat sheet membranes were fabricated in different ratios (1:1:1, 1:1:2, 2:1:1, 2:1:2, 1:2:1, 2:2:1) in a polar solvent of N,N'-dimethylformamide (DMF) using the most popular phase inversion method. Nanochitosan was prepared by the ionotropic gelation method and its average particle size has been analyzed using Dynamic Light Scattering (DLS) method. The effect of blending of the three polymers was investigated using FTIR and XRD studies. FTIR results confirmed the formation of well-blended membranes and the XRD analysis revealed enhanced amorphous nature of the membrane ratio 2:1:2. DSC study was conducted to find out the thermal behavior of the blend membranes and the results clearly indicated good thermal stability and single glass transition temperature (Tg) of all the prepared membranes. Asymmetric nature and rough surface morphology was confirmed using SEM analysis. From the results it was evident that the blending of the polymers with higher concentration of nanochitosan can alter the nature of the resulting membranes to a greater extent and thus amorphous membranes were obtained with good miscibility and compatibility.
Developmental Venous Anomalies Mimicking Neoplasm on 11C-Methionine PET and DSC Perfusion MRI.[Pubmed:28319499]
Clin Nucl Med. 2017 May;42(5):e275-e276.
Elevated relative cerebral blood volume on perfusion MRI and increased uptake on C-methionine PET can be used to diagnose and guide biopsy of brain tumors but are not specific. We report increased uptake on C-methionine PET associated with 4 developmental venous anomalies (DVAs) in 3 children with brain tumors, which could potentially mimic tumor and misdirect biopsy. Because DVAs are not readily visible on CT, prevention of misdirected biopsy in patients with focally elevated C-methionine uptake and relative cerebral blood volume relies on close correlation with contrast-enhanced anatomic MRI to exclude DVA or other nonneoplastic etiology.
Thermal effects in guest-host systems: [Zn2 (bdc)(S-lac)(dmf)]*PhEtOH: A DSC and NMR study.[Pubmed:28370482]
Chirality. 2017 Mar;29(3-4):130-133.
Differential scanning calorimetry and nuclear magnetic resonance were used to investigate thermal effects in the guest-host systems where homochiral metal-organic sorbent [Zn2 (bdc)(S-lac)(dmf)] is considered as a host while 1-phenylethanol enantiomers and their racemic mixture serve as guest molecules. A maximum energy gain from the guest-host interaction was observed in the system with the racemic mixture. The effect of host-guest recognition was revealed for the case of the host and guest having a similar type of chirality in the presence of antipode guest molecules.


