Cudratricusxanthone ACAS# 740810-42-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 740810-42-8 | SDF | Download SDF |
| PubChem ID | 11153672 | Appearance | Powder |
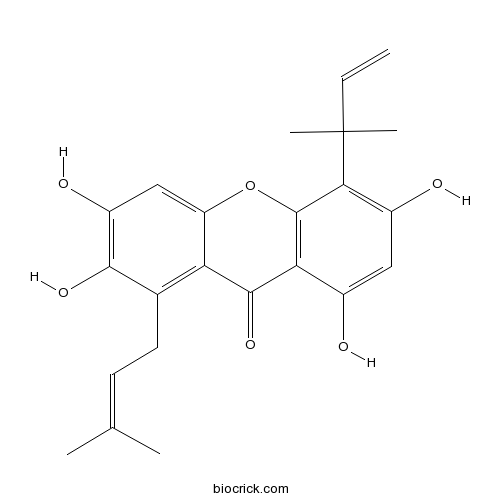
| Formula | C23H24O6 | M.Wt | 396.43 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,3,6,8-tetrahydroxy-5-(2-methylbut-3-en-2-yl)-1-(3-methylbut-2-enyl)xanthen-9-one | ||
| SMILES | CC(=CCC1=C2C(=CC(=C1O)O)OC3=C(C2=O)C(=CC(=C3C(C)(C)C=C)O)O)C | ||
| Standard InChIKey | FUEJTEBWTNXAPG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H24O6/c1-6-23(4,5)19-14(25)9-13(24)18-21(28)17-12(8-7-11(2)3)20(27)15(26)10-16(17)29-22(18)19/h6-7,9-10,24-27H,1,8H2,2-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cudratricusxanthone A has potent anti-proliferative, antioxidative, and monoamine oxidase inhibitory effects, it also possesses anti-cancer activities and provide a basis for developing effective therapeutic agents to inhibit growth and metastasis of breast cancer. Cudratricusxanthone A inhibits lipopolysaccharide-induced neuroinflammation through inhibition of NF-κB and p38 MAPK pathways in BV2 microglia. Cudratricusxanthone A has antiplatelet, anticoagulant, and profibrinolytic activities, it reversibly inhibits CYP1A2, 2C8, and 2C9. It also exhibits the significant hepatoprotective effect on nitrofurantoin-induced cytotoxicity in human liver-derived Hep G2 cells. |
| Targets | COX | NOS | PGE | TNF-α | IL Receptor | p65 | NF-kB | IkB | p38MAPK | ERK | P450 (e.g. CYP17) | ROS | IFN-γ | JAK | STAT | IKK |
| In vitro | A Prenylated Xanthone, Cudratricusxanthone A, Isolated from Cudrania tricuspidata Inhibits Lipopolysaccharide-Induced Neuroinflammation through Inhibition of NF-κB and p38 MAPK Pathways in BV2 Microglia.[Pubmed: 27649130]Molecules. 2016 Sep 16;21(9).
Cudrania tricuspidata Bureau (Moraceae) is an important source of traditional Korean and Chinese medicines used to treat neuritis and inflammation. Cudratricusxanthone A (1), a prenylated xanthone, isolated from C. tricuspidata, has a variety of biological and therapeutic activities. In vitro inhibition of human cytochrome P450 by cudratricusxanthone A.[Pubmed: 25936586 ]Food Chem Toxicol. 2015 Jul;81:171-5.Cudratricusxanthone A (CTXA) isolated from the roots of Cudrania tricuspidata Bureau (Moraceae) has several biological activities, including hepatoprotective, neuroprotective, anti-inflammatory, monoamine oxidase inhibitory, and antithrombotic activities. Hepatoprotective constituents of Cudrania tricuspidata.[Pubmed: 15742807]Arch Pharm Res. 2005 Jan;28(1):44-8.
|
| In vivo | Antiplatelet, anticoagulant, and profibrinolytic activities of cudratricusxanthone A.[Pubmed: 24234914]Arch Pharm Res. 2014 Aug;37(8):1069-78.Cudratricusxanthone A (CTXA), a natural bioactive compound extracted from the roots of Cudrania tricuspidata Bureau, is known to possess hepatoprotective, antiproliferative and anti-inflammatory activities. However, antiplatelet, anticoagulant, and profibrinolytic properties have not been studied.
|
| Kinase Assay | Cudratricusxanthone A protect pancreatic beta cells from cytokines-mediated toxicity through the inhibition of NF-κB and STAT pathways.[Pubmed: 24768584 ]Int Immunopharmacol. 2014 Jul;21(1):26-33.Cudratricusxanthone A (CTXA) has an isoprenylated xanthone skeleton that is known to exert a variety of biological activities, including anti-inflammatory, neuroprotective, hepatoprotective, anti-proliferative, and mono-amine oxidase inhibitory effects. |
| Cell Research | Cudraticusxanthone A isolated from the roots of Cudrania tricuspidata inhibits metastasis and induces apoptosis in breast cancer cells.[Pubmed: 27586822 ]J Ethnopharmacol. 2016 Dec 24;194:57-62.
The roots of Cudrania tricuspidata is a deciduous tree found in Korea, China, and Japan. C. tricuspidata contains an abundance of various minerals, B vitamins, and flavonoids to help prevent diverse cancers. Cudratricusxanthone A (CTXA), a compound isolated from the roots of C. tricuspidata, has potent anti-proliferative, antioxidative, and monoamine oxidase inhibitory effects.
In the present study, Cudratricusxanthone A (CTXA) is a xanthone isolated from the bioassay-guided fractionation of the EtOH extract of C. tricuspidata with strong anti-cancer activity in breast cancer cells. The effect of CTXA on cell migration and apoptosis were evaluated in the MCF-7 and MDA-MB-231 human breast carcinoma cell lines.
|

Cudratricusxanthone A Dilution Calculator

Cudratricusxanthone A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5225 mL | 12.6126 mL | 25.2251 mL | 50.4503 mL | 63.0628 mL |
| 5 mM | 0.5045 mL | 2.5225 mL | 5.045 mL | 10.0901 mL | 12.6126 mL |
| 10 mM | 0.2523 mL | 1.2613 mL | 2.5225 mL | 5.045 mL | 6.3063 mL |
| 50 mM | 0.0505 mL | 0.2523 mL | 0.5045 mL | 1.009 mL | 1.2613 mL |
| 100 mM | 0.0252 mL | 0.1261 mL | 0.2523 mL | 0.5045 mL | 0.6306 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Macamide B
Catalog No.:BCN1366
CAS No.:74058-71-2
- Ketanserin
Catalog No.:BCC5050
CAS No.:74050-98-9
- Lancifolin C
Catalog No.:BCN2019
CAS No.:74048-71-8
- Enoxacin (Penetrex)
Catalog No.:BCC3775
CAS No.:74011-58-8
- p-Hydroxy-cinnamic acid
Catalog No.:BCN5027
CAS No.:7400-08-0
- Mosloflavone
Catalog No.:BCN6796
CAS No.:740-33-0
- L-Arginine
Catalog No.:BCN2691
CAS No.:74-79-3
- Ethambutol
Catalog No.:BCC5195
CAS No.:74-55-5
- Kamebakaurin
Catalog No.:BCN8040
CAS No.:73981-34-7
- Cilostazol
Catalog No.:BCC2291
CAS No.:73963-72-1
- 7,8-Dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one
Catalog No.:BCC8774
CAS No.:73942-87-7
- Isolimonexic acid
Catalog No.:BCN7141
CAS No.:73904-93-5
- ACV 1
Catalog No.:BCC5989
CAS No.:740980-24-9
- Ketorolac
Catalog No.:BCC5190
CAS No.:74103-06-3
- Ketorolac tromethamine salt
Catalog No.:BCC4431
CAS No.:74103-07-4
- SKF 83822 hydrobromide
Catalog No.:BCC7252
CAS No.:74115-10-9
- Norjuziphine
Catalog No.:BCN3367
CAS No.:74119-87-2
- DSC
Catalog No.:BCC2800
CAS No.:74124-79-1
- Bisdethiobis(methylthio)gliotoxin
Catalog No.:BCN7351
CAS No.:74149-38-5
- Pimobendan
Catalog No.:BCC2294
CAS No.:74150-27-9
- 2,3-Dehydrokievitone
Catalog No.:BCN4294
CAS No.:74161-25-4
- R547
Catalog No.:BCC3927
CAS No.:741713-40-6
- Haginin A
Catalog No.:BCN6861
CAS No.:74174-29-1
- Cyclokievitone
Catalog No.:BCC8159
CAS No.:74175-82-9
Hepatoprotective constituents of Cudrania tricuspidata.[Pubmed:15742807]
Arch Pharm Res. 2005 Jan;28(1):44-8.
Phytochemical investigation of the MeOH extract of the root barks of Cudrania tricuspidata Bureau (Moraceae), as guided by hepatoprotective activity in vitro, furnished four isoprenylated xanthones, Cudratricusxanthone A (1), cudraxanthone L (2), cudratricusxanthone E (3), and macluraxanthone B (4). All of these compounds showed the significant hepatoprotective effect on tacrine-induced cytotoxicity in human liver-derived Hep G2 cells. Compounds 1, 2, and 4 also exhibited the significant hepatoprotective effect on nitrofurantoin-induced cytotoxicity in human liver-derived Hep G2 cells.
In vitro inhibition of human cytochrome P450 by cudratricusxanthone A.[Pubmed:25936586]
Food Chem Toxicol. 2015 Jul;81:171-175.
Cudratricusxanthone A (CTXA) isolated from the roots of Cudrania tricuspidata Bureau (Moraceae) has several biological activities, including hepatoprotective, neuroprotective, anti-inflammatory, monoamine oxidase inhibitory, and antithrombotic activities. In this study, we investigated the potential herb-drug interaction of CTXA and nine cytochrome P450 (CYP) isoforms in pooled human liver microsomes (HLMs) using a cocktail probe assay. CTXA reversibly inhibited the CYP1A2-catalyzed phenacetin O-deethylation, CYP2C8-catalyzed paclitaxel 6-hydroxylation, and CYP2C9-catalyzed diclofenac 4'-hydroxylation with half-maximal inhibitory concentration (IC50) values of 3.9, 4.7, and 2.9 microM, respectively. The IC50 values did not change under different preincubation conditions. CTXA showed marked dose-dependent, but not time-dependent, inhibition of CYP1A2 and 2C9 activities in HLMs. Dixon plots showed typical competitive inhibition of CYP1A2 and CYP2C9 with Ki values of 1.3 and 1.5 microM, respectively. Further, CTXA inhibited CYP2C8 in a non-competitive manner with a Ki value of 2.2 microM. Our results showed that CTXA reversibly inhibits CYP1A2, 2C8, and 2C9.
Antiplatelet, anticoagulant, and profibrinolytic activities of cudratricusxanthone A.[Pubmed:24234914]
Arch Pharm Res. 2014 Aug;37(8):1069-78.
Cudratricusxanthone A (CTXA), a natural bioactive compound extracted from the roots of Cudrania tricuspidata Bureau, is known to possess hepatoprotective, antiproliferative and anti-inflammatory activities. However, antiplatelet, anticoagulant, and profibrinolytic properties have not been studied. The anticoagulant activities of CTXA were measured by monitoring activated partial thromboplastin-time (aPTT), prothrombin time (PT), and the activities of cell-based thrombin and activated factor X (FXa). The effects of CTXA on the expressions of plasminogen activator inhibitor type 1 (PAI-1) and tissue-type plasminogen activator (t-PA) were also tested in tumor necrosis factor-alpha (TNF-alpha) activated human umbilical vein endothelial cells. Our data showed that CTXA inhibited thrombin-catalyzed fibrin polymerization and platelet aggregation, prolonged aPTT and PT significantly and inhibited the activities and production of thrombin and FXa. CTXA prolonged in vivo bleeding time and inhibited TNF-alpha induced PAI-1 production. Furthermore, PAI-1/t-PA ratio was significantly decreased by CTXA. Collectively, these results indicate that CTXA possesses antithrombotic activities and suggest that the current study could provide bases for the development of new anticoagulant agents.
A Prenylated Xanthone, Cudratricusxanthone A, Isolated from Cudrania tricuspidata Inhibits Lipopolysaccharide-Induced Neuroinflammation through Inhibition of NF-kappaB and p38 MAPK Pathways in BV2 Microglia.[Pubmed:27649130]
Molecules. 2016 Sep 16;21(9). pii: molecules21091240.
Cudrania tricuspidata Bureau (Moraceae) is an important source of traditional Korean and Chinese medicines used to treat neuritis and inflammation. Cudratricusxanthone A (1), a prenylated xanthone, isolated from C. tricuspidata, has a variety of biological and therapeutic activities. The goal of this study was to examine the effects of compound 1 on neuroinflammation and characterize its mechanism of action in lipopolysaccharide (LPS)-stimulated BV2 microglia. Cudratricusxanthone A (1) suppressed the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 enzymes and decreased the production of iNOS-derived nitric oxide and COX-2-derived prostaglandin E2 in LPS-stimulated mouse BV2 microglia. The compound also decreased tumor necrosis factor-alpha, interleukin (IL)-1beta, and IL-12 production; inhibited the phosphorylation and degradation of IkappaB-alpha; and blocked the nuclear translocation of p50 and p65 in mouse BV2 microglia induced by LPS. Cudratricusxanthone A (1) had inhibitory effects on nuclear factor kappa B DNA-binding activity. Additionally, it inhibited the p38 mitogen-activated protein kinase signaling pathway. Our data suggests that Cudratricusxanthone A (1) may be a useful therapeutic agent in the treatment of neurodegenerative diseases caused by neuroinflammation.
Cudratricusxanthone A protect pancreatic beta cells from cytokines-mediated toxicity through the inhibition of NF-kappaB and STAT pathways.[Pubmed:24768584]
Int Immunopharmacol. 2014 Jul;21(1):26-33.
Cudratricusxanthone A (CTXA) has an isoprenylated xanthone skeleton that is known to exert a variety of biological activities, including anti-inflammatory, neuroprotective, hepatoprotective, anti-proliferative, and mono-amine oxidase inhibitory effects. In this study, we investigated the effect of CTXA on IL-1beta (5 ng/ml) and IFN-gamma (100 U/ml)-induced beta-cell damage. Pre-treatment with CTXA increased the viability and reactive oxygen species (ROS) inhibition of cytokine-treated RINm5F cells at concentrations of 1-10 muM. CTXA prevented nitric oxide (NO) production, and this effect was correlated with reduced levels of protein and mRNA expression of inducible nitric oxide synthase (iNOS). The molecular mechanism by which CTXA inhibits iNOS gene expression appeared to involve the inhibition of NF-kappaB activation. Moreover, pancreatic beta-cells treated with cytokines upregulated the phosphorylation of STAT-1, STAT-3 and STAT-5; however, pretreatment with CTXA attenuated these effects. Additionally, in a second set of experiments in which rat islets were used, the protective effects of CTXA in rat islets were essentially the same as those observed when RINm5F cells were used. CTXA prevented cytokines-induced NO production, iNOS expression, JAK/STAT activation, and NF-kappaB activation and inhibition of glucose-stimulated insulin secretion (GSIS). Collectively, these results suggest that CTXA can be used for the prevention of functional beta-cell damage.
Cudraticusxanthone A isolated from the roots of Cudrania tricuspidata inhibits metastasis and induces apoptosis in breast cancer cells.[Pubmed:27586822]
J Ethnopharmacol. 2016 Dec 24;194:57-62.
ETHNOPHARMACOLOGICAL RELEVANCE: The roots of Cudrania tricuspidata is a deciduous tree found in Korea, China, and Japan. C. tricuspidata contains an abundance of various minerals, B vitamins, and flavonoids to help prevent diverse cancers. Cudratricusxanthone A (CTXA), a compound isolated from the roots of C. tricuspidata, has potent anti-proliferative, antioxidative, and monoamine oxidase inhibitory effects. AIM OF THE STUDY: In the present study, Cudratricusxanthone A (CTXA) is a xanthone isolated from the bioassay-guided fractionation of the EtOH extract of C. tricuspidata with strong anti-cancer activity in breast cancer cells. The effect of CTXA on cell migration and apoptosis were evaluated in the MCF-7 and MDA-MB-231 human breast carcinoma cell lines. MATERIALS AND METHODS: Effects of CTXA on phorbol 12-myristate 13-acetate (PMA)-induced MCF-7 and MDA-MB-231 cells. Flow cytometric measurements of CTXA-induced apoptosis in breast cancer cells. RESULTS: The results show that CTXA gradually reduced viability of the two breast cancer cell lines and induced apoptosis in a concentration-dependent manner. Moreover, CTXA effectively blocked breast cancer cell migration and invasion. CTXA decreased the expression of matrix metalloproteinase-9, extracellular regulated kinases 1 and 2 and phosphorylation of the inhibitor IkappaBalpha in the MCF-7 and MDA-MB-231 cell lines. CONCLUSIONS: Collectively, these results indicate that CTXA possesses anti-cancer activities and provide a basis for developing effective therapeutic agents to inhibit growth and metastasis of breast cancer.


