DMAPCAS# 1122-58-3 |
- 7-Chlorokynurenic acid sodium salt
Catalog No.:BCC7757
CAS No.:1263094-00-3
- TFB-TBOA
Catalog No.:BCC5919
CAS No.:480439-73-4
- Dihydrokainic acid
Catalog No.:BCC6556
CAS No.:52497-36-6
- L-(-)-threo-3-Hydroxyaspartic acid
Catalog No.:BCC6565
CAS No.:7298-99-9
- WAY 213613
Catalog No.:BCC7442
CAS No.:868359-05-1
- LDN 212320
Catalog No.:BCC6361
CAS No.:894002-50-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1122-58-3 | SDF | Download SDF |
| PubChem ID | 2735020 | Appearance | Powder |
| Formula | C7H10N2 | M.Wt | 122.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | N,N-dimethylpyridin-1-ium-4-amine | ||
| SMILES | CN(C)C1=CC=[NH+]C=C1 | ||
| Standard InChIKey | VHYFNPMBLIVWCW-UHFFFAOYSA-O | ||
| Standard InChI | InChI=1S/C7H10N2/c1-9(2)7-3-5-8-6-4-7/h3-6H,1-2H3/p+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

DMAP Dilution Calculator

DMAP Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.1833 mL | 40.9165 mL | 81.8331 mL | 163.6661 mL | 204.5827 mL |
| 5 mM | 1.6367 mL | 8.1833 mL | 16.3666 mL | 32.7332 mL | 40.9165 mL |
| 10 mM | 0.8183 mL | 4.0917 mL | 8.1833 mL | 16.3666 mL | 20.4583 mL |
| 50 mM | 0.1637 mL | 0.8183 mL | 1.6367 mL | 3.2733 mL | 4.0917 mL |
| 100 mM | 0.0818 mL | 0.4092 mL | 0.8183 mL | 1.6367 mL | 2.0458 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
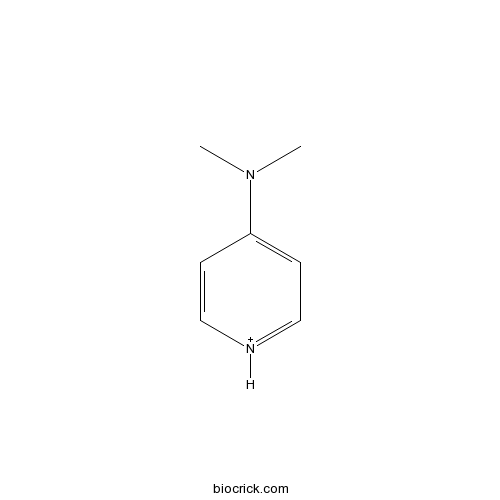
DMAP
- Ikshusterol 3-O-glucoside
Catalog No.:BCN6001
CAS No.:112137-81-2
- (R)-(-)-Modafinic acid
Catalog No.:BCC5157
CAS No.:112111-45-2
- (S)-(+)-Modafinic acid
Catalog No.:BCC5158
CAS No.:112111-44-1
- 3-Hydroxy-2-methylpyridine
Catalog No.:BCN8162
CAS No.:1121-25-1
- Endoxifen
Catalog No.:BCC7761
CAS No.:112093-28-4
- p-Vinylphenyl O-[beta-D-apiofuranosyl-(1-6)]-beta-D-glucopyranoside
Catalog No.:BCN1619
CAS No.:112047-91-3
- OctMAB
Catalog No.:BCC7893
CAS No.:1120-02-1
- Docosanoic acid
Catalog No.:BCC8952
CAS No.:112-85-6
- Oleic acid
Catalog No.:BCN7159
CAS No.:112-80-1
- Methyl linoleate
Catalog No.:BCN8137
CAS No.:112-63-0
- Methyl Oleate
Catalog No.:BCN8306
CAS No.:112-62-9
- Methyl Stearate
Catalog No.:BCN8309
CAS No.:112-61-8
- Pam3CSK4
Catalog No.:BCC6245
CAS No.:112208-00-1
- 14,15-Didehydrovincamenine
Catalog No.:BCN6002
CAS No.:112219-48-4
- 16-O-Methyl-14,15-didehydroisovincanol
Catalog No.:BCN1618
CAS No.:112237-71-5
- Stigmastane-3,6-diol
Catalog No.:BCN6003
CAS No.:112244-29-8
- (S)-tert-Leucinol
Catalog No.:BCN8367
CAS No.:112245-13-3
- 20(R)-Ginsenoside Rh2
Catalog No.:BCN2484
CAS No.:112246-15-8
- H-Asp(OcHex)-OH
Catalog No.:BCC2887
CAS No.:112259-66-2
- BRL 52537 hydrochloride
Catalog No.:BCC6751
CAS No.:112282-24-3
- WAY-262611
Catalog No.:BCC5507
CAS No.:1123231-07-1
- DMPQ dihydrochloride
Catalog No.:BCC6977
CAS No.:1123491-15-5
- XL765
Catalog No.:BCC2060
CAS No.:1123889-87-1
- Tetramethylpyrazine
Catalog No.:BCN1008
CAS No.:1124-11-4
Looking for new antiplasmodial quinazolines: DMAP-catalyzed synthesis of 4-benzyloxy- and 4-aryloxy-2-trichloromethylquinazolines and their in vitro evaluation toward Plasmodium falciparum.[Pubmed:27155463]
Eur J Med Chem. 2016 Aug 25;119:34-44.
A DMAP catalyzed synthesis of new 4-benzyloxy- and 4-aryloxy-2-trichloromethylquinazolines was studied, in a view to react 4-chloroquinazolines with poorly nucleophilic alcohols such as benzylic alcohols, via a simple and cheap SNAr reaction approach. A fast (1 h) general operating procedure, affording good reaction yields, was achieved under microwave irradiation. Thus, a series of 35 molecules was obtained and evaluated in vitro on the K1 multi-resistant Plasmodium falciparum strain, in parallel with a cytotoxicity assessment on the human HepG2 cell line. 5 hit-molecules were identified, presenting both promising antiplasmodial activity (1.5 muM < IC50 < 2 muM) and low cytotoxicities (25 muM < CC50 < 45 muM). Apart for 2 molecules, the global series displayed a satisfying solubility in the aqueous biological media. Structure-activity relationships showed that the molecules presenting a benzyloxy moiety were less cytotoxic than the ones bearing a phenoxy moiety at position 4 of the quinazoline ring. It also appeared that the introduction of a heteroaryl moiety afforded inactive compounds. Finally, the most active and selective molecules (Selectivity Index = 22-27) were the ones presenting either an unsubstituted benzyloxy group or a phenoxy group, this last bearing a p-bromo or an o-acetyl substituent.
First DMAP-mediated direct conversion of Morita-Baylis-Hillman alcohols into gamma-ketoallylphosphonates: Synthesis of gamma-aminoallylphosphonates.[Pubmed:28144364]
Beilstein J Org Chem. 2016 Dec 30;12:2906-2915.
An efficient synthesis of a series of gamma-ketoallylphosphonates through a direct conversion of both primary and secondary Morita-Baylis-Hillman (MBH) alcohols by trialkyl phosphites with or without DMAP, used as additive, and under solvent-free conditions, is described herein for the first time. Subsequently, a highly regioselective Luche reduction of the primary phosphonate 2a (R = H) gave the corresponding gamma-hydroxyallylphosphonate 5 that further reacted with tosylamines in the presence of diiodine (15 mol %) as a catalyst, affording the corresponding SN2-type products 6a-d in 63 to 70% isolated yields. Alternatively, the alcohol 5 produced the corresponding acetate 7 which, mediated by Ce(III), was successfully converted into the corresponding gamma-aminoallylphosphonates 8a-d.
Beyond a Protecting Reagent: DMAP-Catalyzed Cyclization of Boc-Anhydride with 2-Alkenylanilines.[Pubmed:27163704]
J Org Chem. 2016 Jun 3;81(11):4645-53.
A novel rapid synthesis of quinolines from 2-alkenylanilines has been described; the reaction involves an unexpected DMAP-catalyzed cyclization of 2-alkenylanilines with di-tert-butyl dicarbonate (Boc2O, 2.0 equiv), and a series of tert-butyl quinolin-2-yl carbonate with various functional groups have been synthesized in good yields under mild conditions. Furthermore, the tert-butyl quinolin-2-yl carbonate can be easily converted into corresponding quinolinones and 2-(pseudo)haloquinolines.
Development of a Chiral DMAP Catalyst for the Dynamic Kinetic Resolution of Azole Hemiaminals.[Pubmed:28060519]
J Org Chem. 2017 Jan 20;82(2):869-886.
A new catalyst for the dynamic kinetic resolution of azole hemiaminals has been developed using late-stage structural modifications of the tert-leucinol-derived chiral subunit of DMAP species.


